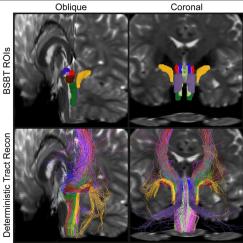
A few years ago, working with mice, Gloria Choi and colleagues discovered a molecular and circuit basis for how certain genetic abnormalities or maternal infection during pregnancy may lead to an increased risk of autism-like social behavior deficits in offspring. But a huge question remained: Are the processes occurring in humans?
There were certainly reasons to think so. The original association between maternal infection and autism risk in progeny had been established in humans and the genetic anomalies engineered into the mice also were first discovered in patients. Choi’s mouse studies pinpointed aberrant development and hyperactivity in the S1DZ area of the mouse somatosensory cortex. S1DZ is analogous to, but not exactly the same as, the “3a” region of the same cortex in humans. To know for sure whether the mechanisms of action in mice are also at play in humans would require studying children, not mouse
pups.
Starting a human study is not easy but fortunately, one of Korea’s foremost autism physicians was visiting around the corner at the Broad Institute. Dr. Hyo-Won Kim took the short walk to Choi’s office to meet about forging the collaboration needed to answer the question. Today they are enrolling patients at Asan Medical Center in Seoul to conduct MRI scans that will confirm or refute the hypothesis that hyperactivity in the 3a region is afoot in human autism patients. If that proves true, then treatments to reduce hyperactivity in the region might provide a meaningful benefit. For instance, Choi has found that the immune system cytokine IL-17a can reduce hyperactivity in S1DZ.
Though Choi had never launched a human study before, she says she felt compelled to seize the opportunity.
“The brains of rodents are very different from the brains of humans,” said Choi, Mark Hyman Jr. Associate Professor of Neurobiology. “In order to make these findings worth it and mean something, we have to show it also happens in humans, too. I had no other choice but to pursue it.”
Indeed though The Picower Institute focuses on fundamental neuroscience research, several members of the faculty, upon making discoveries with clear clinical potential, have pursued a variety of approaches to ensure that the medical value of the discoveries is clinically investigated in humans.
“All routes are open to us,” said Picower Professor Mark Bear, who has both launched companies and forged clinical collaborations to develop therapies and diagnostics based on his research.
Testing in-house
For Choi a serendipitous connection became an external clinical collaboration, but for Picower Professor Li-Huei Tsai, a similar circumstance led to a different approach: building an in-house clinical testing team.
In 2016 Tsai’s lab showed in mice that stimulating 40Hz “gamma” frequency brain rhythms with light flashing at that same frequency could reduce the buildup of Alzheimer’s pathology in the brains of mice. This caught the attention of neurologist Diane Chan who had been in touch with the lab about a fellowship.
“She became very excited about translating the animal results to human subjects,” Tsai said. “It was perfect timing”
And so Tsai and Chan created a group within the Tsai lab, the “Human Gamma Team,” to develop 40Hz stimulation devices, recruit volunteers, and design and carry out clinical studies of the potential therapy. One crucial attribute that made doing so easier is that the method is completely non-invasive, unlike a drug or an implanted device.
“There are so many treatments that cure Alzheimer’s in mouse models but essentially all of them fail in humans,” Tsai said. “In the end how relevant is the scientific discovery in the most strict sense and critical sense? It has to be validated in humans.”
Last year they posted a pre-print study showing encouraging preliminary results from combining light and sound stimulation in human patients in a double-blinded, controlled pilot study. Compared to people who started out receiving the placebo version, people who got 40Hz stimulation for an hour a day for several weeks showed reduced atrophy of the hippocampus, better synchrony and connectivity between brain regions, improved sleep, and better performance on a face-name association memory test.
It was a huge change for the Tsai lab to expand to clinical studies, but like Choi, Tsai wanted to directly test her discovery’s medical potential.
“There are so many treatments that cure Alzheimer’s in mouse models but essentially all of them fail in humans,” Tsai said. “In the end how relevant is the scientific discovery in the most strict sense and critical sense? It has to be validated in humans.”
The team is now embarking on new studies. Can the stimulation prevent the onset of cognitive impairment? Can it benefit patients with Parkinson’s disease? What about Down syndrome? With an in-house, physician-led team, Tsai can readily ask these new questions even as the rest of her lab continues more fundamental Alzheimer’s related research.
Founding a new center
As an anesthesiologist practicing at Massachusetts General Hospital (MGH), Emery N. Brown, Edward Hood Taplin Professor of Medical Engineering and Computational Neuroscience, has a built-in opportunity to translate his fundamental neuroscience and statistics research to clinical practice, but for a long time he kept the two worlds separate. Around 2005, though, he started to apply his research analyzing neuroscience data to his work in the operating room, breaking through a
traditional barrier.
“Anesthesia is not viewed as part of neuroscience,” he said. “It’s viewed as a part of clinical pharmacology.”
But a significant part of anesthesiology is controlling the brain’s arousal state and the body’s pain state, not just getting chemicals into cells so they’ll be metabolized. Brown’s studies have shown that different anesthetic drugs produce distinct “signature” patterns of rhythms when people are unconscious. The patterns vary characteristically with drug mechanism of
action, dose, the patient’s age and the patient’s state of health. These insights allowed him to use EEG-based brain rhythm monitoring in the OR to directly assess the unconsciousness of his patients. In this way he can more precisely control drug dosing so that patients experience the benefits of general anesthesia without any overdosing that could cause post-operative side-effects like nausea, vomiting or brain dysfunction.
Understanding how anesthesia and arousal control work on a systems neuroscience level opens up many possibilities for improving patient care, Brown says. To explore those he is working to found a new center spanning MIT and MGH called the Brain Arousal State Control Innovation Center, or BASCIC. He and colleagues envision no less than six basic science or clinical studies based on fundamental neuroscience research: Waking patients after anesthesia by activating specific arousal pathways; testing a specific anesthetic as a sleep aid; using anesthetics to develop a treatment for Alzheimer’s; developing a closed-loop system for measuring brain rhythms and automatically adjusting anesthetic dose; testing an anesthetic as an antidepressant; and treating brain arousal pathways to stimulate coma recovery.
BASCIC, as a center, could help to seed fund and administrate all these studies.
Leveraging the marketplace
Bear, too, has been working to test and develop fundamental discoveries with therapeutic potential for more than a decade. Sometimes the marketplace is the best place to try that, especially given the significant financial resources that late-stage trials require. Bear is well known, for instance, for having co-founded Seaside Therapeutics to spur development and testing of a promising treatment his lab discovered for Fragile X syndrome, a genetically caused form of autism. Though that specific effort fell just short in a high-profile phase III trial, he has recently started a new company, Allos Pharma Inc., to continue development and testing.
In parallel with her in-house clinical testing, Tsai also co-founded Cognito Therapeutics to spur larger-scale testing and development of 40Hz sensory stimulation. Earlier this year the company launched phase III clinical trials.
Bear is also now weighing how best to bring to the clinic another therapeutic strategy developed in his lab. They have shown in multiple animal models that the common vision disorder amblyopia can be overcome, even in adulthood, by briefly anesthetizing the unaffected eye. That essentially “reboots” the level of visual activity needed to strengthen connections in the brain serving the impaired eye. In the year since his lab published highly encouraging results, he has heard strong
interest both from eye specialists and also from an ophthalmology company about bringing it to people. He has spoken extensively with the potential clinical collaborators and has met twice with the company.
“We have a number of irons in the fire,” Bear said.
To advance the therapy, the company would license the patent from MIT and develop and test a treatment based on it. Tsai has done that with another of her Alzheimer’s discoveries. Inhibiting an enzyme called HDAC2, which becomes overactive in Alzheimer’s, restores the ability of neurons to forge connections that sustain learning and memory. That was licensed by a company called Rodin Therapeutics.
Newton Professor Mriganka Sur has also watched a company advance one of his therapeutically relevant discoveries. Over 15 years ago, his lab showed that IGF-1 is required to promote maturation of synaptic connections in the cerebral cortex. In 2009, studying mice modeling the mutation that causes Rett syndrome, they showed that the disease causes the brain’s connections to remain immature into adulthood, but administering the peptide form of IGF-1 had substantial therapeutic benefits for neurological and non-neurological symptoms of the disorder. A company developed an oral formulation to prolong the molecule’s bioavailability, which Sur supported because of the urgent need for a therapy to reach patients. Starting in 2014, the company successfully completed phase I and II trials in human patients, and late last year, they announced success of a phase III trial. In July this year, they asked the FDA to approve the treatment.
That decision is pending, but if the FDA says yes, IGF-1 for Rett syndrome could become the first example of a Picower-discovered therapy to turn into a medicine, going all the way down the long, complicated road from lab bench to patient bedside.
In different ways, Picower faculty have advanced and enabled clinical application of fundamental work.
This story originally appeared in the Fall 2022 version of The Picower Institute's print newsletter. Subscribe for free.