None of the faculty members in The Picower Institute is an “autism researcher,” but The Institute has been advancing research on autism spectrum disorders for nearly 20 years. Five labs are doing autism research right now. How can this be? Picower Institute scientists seek to discover the fundamental mechanisms that make the brain work. That turns out to be a fruitful—and maybe essential—way to improve understanding of how it sometimes works differently.
“You can't solve a brain disorder by just studying the disorder,” said Newton Professor Mriganka Sur, a Picower Institute faculty member who directs MIT’s Simons Center for the Social Brain, a major hub for Boston-area autism research. “It will be difficult to understand the disorder unless that understanding is built on a lot of basic biology.”
It’s not a simple challenge. The autism spectrum includes conditions that produce severe intellectual disabilities and others that produce behavioral differences that advocates say merely need to be recognized, rather than medicalized, Sur said. What unifies the spectrum are difficulties with social behavior, restricted interests, and repetitive behaviors. The U.S. Centers for Disease Control estimates that 1 in 31 American eight-year-olds had an autism diagnosis in 2022.
The causes of ASDs are also complex. About 30 years ago scientists began tracing some profound ASDs, such as Fragile X syndrome, directly to single genes. They have since accumulated evidence associating hundreds of genes with increasing likelihood of autism. Some non-genetic, or “environmental,” factors, such as maternal infection during fetal development, also play a role.
Given the complexities, research must incorporate many perspectives and scales, ranging from the molecules that help neurons communicate via “synapse” connections to the cognition produced by the coordinated neural activity of millions of cells. That’s part of what has made MIT’s brain science community, and in particular The Picower Institute for Learning and Memory, such a productive contributor to the global autism research enterprise, said Sur who also led MIT’s Department of Brain and Cognitive Sciences from 1997 to 2012.
“The disorder manifests all the way from genes, proteins, synapses to circuits and behavior,” he said. “This is the place where we have built a department and these centers that study the brain and mind across so many levels of analysis where autism should be studied.”
Indeed, among the updates below on autism research projects in five Picower labs, each is deeply rooted in a history of fundamental curiosity about brain function. Taken together, the investigations span scales from subcellular structures to wide brain networks.
Connections from connections
Picower Professor Mark Bear’s autism research began with his discovery of molecular mechanisms by which synaptic connections can weaken, a process called “long-term synaptic depression” (LTD). In one mechanism, the neurotransmitter glutamate activates a neural receptor called mGluR5, spurring protein synthesis at the synapse. When Bear learned that the protein missing in fragile X syndrome—called FMRP—restrains protein synthesis, he realized that a potential source of the syndrome’s neurological symptoms could be exaggerated responses to mGluR5 activation. Bear hypothesized that inhibiting protein synthesis at the synapse could be a fragile X treatment strategy.
“You can't solve a brain disorder by just studying the disorder. It will be difficult to understand the disorder unless that understanding is built on a lot of basic biology.” --Mriganka Sur
“I spent a lot of time thinking about the scientific risk of shifting my focus to fragile X,” Bear said. “But the possibility was so exhilarating that we might be able to help kids with fragile X, I decided to jump in with both feet.”
In 2007 his lab showed that genetically knocking down mGluR5 activity significantly improved many symptoms for fragile X mice. In succeeding years, Bear and collaborators sought out, identified and tested several drugs that could inhibit signaling by mGluR5, including in phase II and III clinical trials. His lab also discovered that the mGluR5 pathway mattered not only in fragile X, but also several other ASDs.
In 2011, for instance, his lab showed that mGluR5-related protein synthesis engaged in a molecular tug-of-war with another protein synthesis process driven by other receptors. If the balance moved too far in one way, it led to fragile X, if it went the other way, it led to another ASD: Tuberous Sclerosis (TSC). In a new paper this past February, Bear’s lab discovered a receptor that, when activated, could restore the balance to treat Fragile X mice. The study also implied (but didn’t test) that reducing activation of the receptor might restore the balance in TSC mice.
Meanwhile, Bear identified another drug to treat fragile X, called arbaclofen, that acts to suppress glutamate release. Studies in two mouse models of genetically defined causes of autism, fragile X and chromosome 16p11.2 microdeletion, yielded promising results. He founded a company, Seaside Therapeutics, to run clinical trials in fragile X. The phase 2 and phase 3 studies yielded similarly poignant results: encouraging benefits but near misses of the main behavioral endpoints needed for FDA approvals.
Clinical trial variables such as dose and treatment duration, patient ages and outcome measures, are difficult to guess in advance, and the company ran out of funding to continue. However, many of the lessons from this early work were applied in recent academic trials of arbaclofen in Europe and Canada in patients with ASDs of unknown cause, and the results suggest a broad benefit in social function Bear said. . To raise the investment needed to launch a new U.S. trial, he has formed a new company, Allos Pharmaceuticals.
And in the lab, postdoc and former graduate student Sara Kornfeld-Sylla is studying a potential new way to personalize treatment for fragile X patients. She has documented in both mice and humans atypical brain wave patterns that vary by individual. Kornfeld-Sylla’s research suggests that the signatures could be used as a non-invasive readout to guide how much medicine an individual needs.
Down the hall from Bear’s lab in The Picower Institute is that of Menicon Professor Troy Littleton, whose lab works with fruit flies to study the fundamental protein machinery that enables synapses to form, function and change. Back in 2013 Littleton joined a three-lab Simons Center “targeted project” to investigate how mutations in a gene called Shank3 lead to ASD pathophysiology and to identify treatments. In a 2016 paper, Littleton’s lab showed that the Shank3 protein is necessary for the receiving side of a synaptic connection to incorporate signaling receptors needed for proper formation and maturation. With an eye on a therapeutic strategy, the study demonstrated ways to rescue synapse development.
Now Littleton’s lab has embarked on another autism project. Postdoc Chhavi Sood, a Simons Center Fellow, is looking at a different function of FMRP than Bear’s lab. Her studies focus on a possible role in regulating how proteins are trafficked within neurons to create channels for importing calcium and exporting potassium ions. Those electrochemical dynamics affect how often a cell releases neurotransmitters across a synapse. In fragile X, neurons are too active and Sood’s experiments examining how FMRP mutations affect ion channels have begun to identify clues as to why.
A center and a treatment
When Sur founded the Simons Center in 2012, his goal with the Simons Foundation Autism Research Initiative was to support such projects: multi-lab investigations of major themes and enterprising new ideas among young fellows. Over the last 13 years, the center has not only involved virtually every Picower Institute lab, but also 16 institutions around Boston, yielding about 400 peer-reviewed papers.
Sur’s own research has illuminated several aspects of autism. He has been part of a multi-lab project to evaluate whether marmosets, a small but highly social primate, might be a uniquely informative model organism for autism studies. In a paper earlier this year his lab showed that they make certain predictions much like humans do, which is important because prediction is a cognitive function known to differ in ASDs.
Sur has led pioneering research on an autism-related disorder, Rett syndrome, that originated from his studies of synapses. Caused by mutations in the gene MeCP2, Rett syndrome leads not only to autism symptoms but devastating intellectual, motor and other disabilities. When Sur’s lab in 2006 was studying synaptic proteins, his team found that a protein called IGF-1 was needed for synapses to mature. Based on a 2007 paper that showed that shutting down the MeCP2 gene made mice sick, but even adolescent mice recovered when the gene was turned on, Sur inferred that in Rett syndrome, synapses remain immature but can be induced to mature. Eager to use his insight for impact, he obtained mice modeling Rett syndrome from an MIT colleague and gave them doses of an IGF-1 peptide. In a 2009 study, Sur’s team reported that treated Rett mice improved markedly. His study caught the attention of a company that had an IGF-1 peptide, but hadn’t known to try it with Rett patients. That company went on to conduct successful clinical trials leading to the first-ever FDA-approved Rett syndrome treatment in 2023.
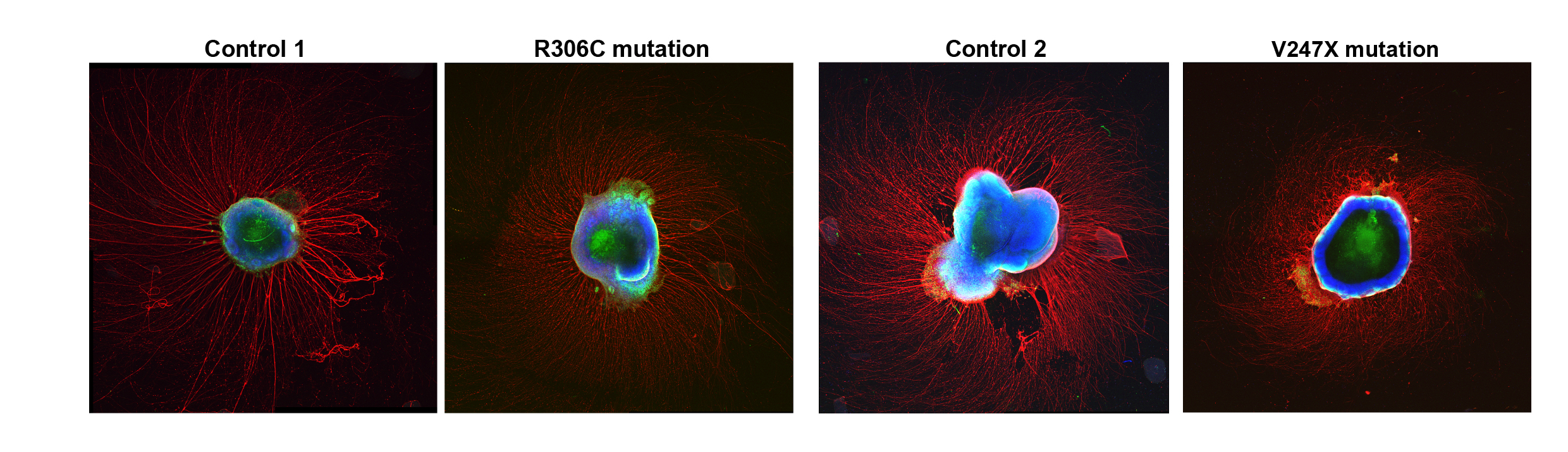
Sur has continued to publish studies revealing how MeCP2 mutations disrupt brain development. A 2017 study found that mutations can lead to misregulation of micro RNAs that in turn hinders the birth of new neurons. Using advanced microscopy techniques, Sur’s lab has also shown that newborn human neurons have trouble migrating to their correct positions in 3D brain tissue cultures. In a new study, online as a preprint, Sur’s lab extends that work by examining neural activity in the 3D cultures. They document how two specific MeCP2 mutations each alter the structure, gene expression and neural activity patterns in the cultures. They show that for each mutation, they could rescue the observed dysfunction the lab. Notably, the used baclofen (related to arbaclofen) for one of those rescues.
Another new project in Sur’s lab focuses on the neural and circuit roots of Rett motor symptoms, focusing on differences in noradrenaline expression and release in the motor cortex.
Immune insights
Sur also collaborates with Picower Institute Associate Professors Gloria Choi and Myriam Heiman and Harvard immunologist Jun Huh on a project to determine whether delivering immune system molecules to the brain could relieve social behavior abnormalities observed in multiple ASDs.
The idea’s origin traces back to Choi and Huh’s days in graduate school at Caltech where, based on observations that maternal infection correlated with increased autism risk, professor Paul Patterson had posited a connection. With Patterson’s hypothesis on her mind, Choi came to MIT in 2013 interested in pursuing studies of how the immune system and central nervous systems interact, a young field called neuroimmunology. Patterson’s hypothesis seemed like a good project to pursue in that larger context.
In a series of papers between 2016 and this past April, Choi and Huh have made huge inroads using mice. Taken together, the studies revealed that in pregnant dams with a particular microbiome, infection can prompt immune cells to produce an excess of the immune system signaling molecule IL-17A. When the molecule reaches a particular surface region of the fetus’s developing brain called S1DZ, that perturbs development leading to excessive neural activity. This abnormality, they showed, later manifests as ASD symptoms in the offspring as adults because S1DZ is involved in circuits that govern social behavior.
The IL-17 research also explained the so-called “fever effect.” Some ASD patients experience social behavior improvements when they are sick. In a 2019 study, the pair discovered that mice affected by maternal infection in utero will produce excess IL-17A when they get an infection as adults. At this later time in life, however, the IL-17A has the effect of calming neural hyperactivity in S1DZ, leading to the improved social behavior. The newest paper shows that IL-17A promotes some S1DZ neurons to make IL-17E, which is actually what does the calming. Meanwhile, they’ve also discovered that a few other ASD mouse models show improved social behavior if either IL-17A or IL-17E is delivered therapeutically (for instance via a nasal spray).
“We started with one model based on an environmental factor (maternal infection) but our findings generalize to other models, including ones based on genetic causes,” Choi said.
Choi and Huh hope to turn their insights from the “fever effect” into an widely applicable therapy. With Sur and Heiman, supported by a Simons Center grant, they are now investigating how IL-17A gets to the brain and whether they can safely manipulate those mechanisms to increase delivery of the molecule when its needed. Complementing that is a project, funded by the Marcus Foundation, to study the fever effect in humans. Working with colleagues at the Lurie Center for Autism at Massachusetts General Hospital they are recruiting autistic volunteers who do or don’t experience the fever effect to provide biological samples and undergo behavioral evaluations. The hope is to rigorously document the fever effect from behavior to molecular correlates to understand more about how it works.
Network broadcasts
Many behaviors, especially cognition, are the products of billions of synapses connecting millions of neurons in multiregional networks. Picower Professor Earl K. Miller is an expert in understanding how these networks operate with the speed and flexibility to enable our intellectual abilities. He’s shown over numerous studies that the brain coordinates this complex neural activity with brain waves of different frequencies, almost like radio broadcasts.
As noted above, scientists hypothesize that making predictions is an ability that differs in ASDs. It may contribute to why many autistic people experience a feeling of sensory overload. Prediction enables the brain to filter out what stimuli are mundane so it can focus on what’s new and salient. In a new Simons Center-funded project, Miller’s lab is part of a team investigating in animals and human volunteers whether the differences in prediction seen in autism are reflected by differences in how the brain employs brainwaves to coordinate its networks. Typically, low-frequency “beta” waves would filter the activity of higher-frequency “gamma” waves that encode new sensory information. Miller’s team hypothesizes that the beta waves are reduced in autistic patients. If that’s true, then a potential therapeutic strategy would be to find ways to amplify beta wave activity.
“If you harness these brain rhythms you can actually treat neuropsychiatric disorders,” Miller said.
Across five labs and many scales, Picower Institute researchers are bringing their expertise about how the brain works to improve understanding and treatment for ASDs.