You don’t look through compound eyes as you fly. Your navigation isn’t aided by four legs, a set of whiskers and a nose millimeters above the ground. You’ve never squirmed through a patch of bacteria to lay your eggs. And you can’t use your umbrella-shaped body to swim, stinging your dinner as you go.
But what you do have are neurons that connect and communicate to endow your nervous system with all its abilities, and so do classic neuroscience model organisms like mice, Drosophila melanogaster fruit flies, and Caenorhabditis elegans worms, and a newcomer, the Clytia hemisphaerica jellyfish. Such species might seem to be curious candidates in which to ask meaningful questions about being human, but because they pack many of the same fundamental features into much simpler and experimentally tractable forms, they are valuable exemplars of nervous system function both in health and disease.
“The history of neuroscience shows that when we study the right animal to ask the right question -- even animals that are very different from humans -- we can reveal fundamental principles of how nervous systems work that are universal,” said Lister Brothers Associate Professor Steven Flavell, whose research employs C. elegans to study how behaviors emerge from neural activity.
Picower Professor Susumu Tonegawa’s 1987 Nobel Prize-winning immunology research involved mice. Picower Professors Li-Huei Tsai and Mark Bear have developed candidate therapies for Alzheimer’s disease and autism in mice. And before the FDA on March 10 approved the first-ever treatment for the devastating neurodevelopmental disorder Rett syndrome, Newton Professor Mriganka Sur had to discover the central role of the protein IGF-1 in mice. Crucially, the protein works the same way in people.
And while fundamental similarities have made many models influential to the point of being indispensable, understanding the many ways organisms differ is valuable, too. Looking across a diversity of nervous systems is the best way biologists can ascertain how they evolved. Learning the specific ways animal models of disease differ from humans can lead to ideas for improving those models and guide experimental interpretations. And studying what’s unique in biology has inspired new technologies. Among many examples, scientists use green fluorescent protein, first discovered in jellyfish, to visualize the expression and location of key proteins in cells – a revolutionary research tool.
“We can learn incredible amounts just from understanding the weird biology that's out there,” said Assistant Professor Brady Weissbourd. ‘Breakthrough technologies come from studying amazing capabilities of organisms.”
Health and disease mechanisms
When a trait is found in multiple species, scientists say it is “conserved,” because after it evolved in some ancestor organism, it stuck around in descendants, likely because it conveyed an important advantage.
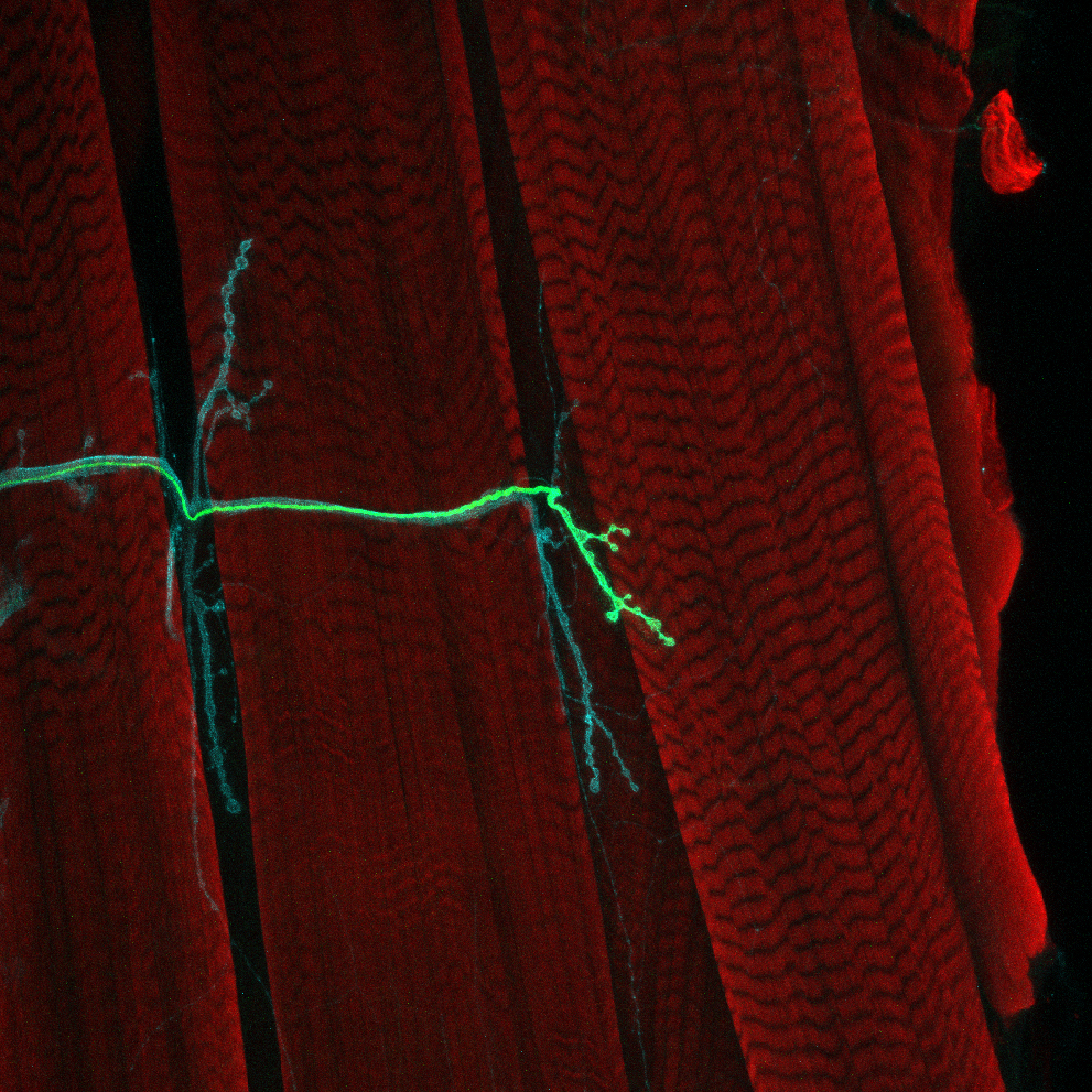
Hundreds of millions of years ago nature evolved a method for neurons to connect and communicate in circuits and networks: releasing chemicals called neurotransmitters across synapses. In the research Menicon Professor Troy Littleton performs in flies to understand how synapses develop and function, almost all the genes and proteins he encounters have direct analogs (or “homologs”) in humans. And because proper synapse function is so essential to brain health, Littleton’s research into the molecular workings of fly synapses has led him to produce many insights about human diseases.
Fruit flies became popular models in neuroscience because they can be bred quickly and raised by the thousands in a lab. They can therefore be induced to rapidly produce genetic mutations that can be very informative about how genes function. If scientists want to know what a gene and the protein it encodes is good for, they can screen their flies until they find ones where it has mutated and see how that aberration affects the fly’s function (nowadays scientists can also directly edit genes, of course).
Using genetic techniques, Littleton and his lab have characterized numerous proteins in synaptic development and function. When a neuron becomes electrically excited enough to send a signal, calcium ions surge into synapses to trigger neurotransmitter release. Among other discoveries, Littleton’s lab has shown that the number of calcium channels at release sites determines their strength and how much neurotransmitter they release, that the synaptic protein Complexin acts to prevent premature release of neurotransmitters until electrical signals are propagated into nerve terminals, and that the calcium-sensor Synaptotagmin 1 activates the process. He’s also discovered that two other key synaptic proteins, Synaptotagmin 7 and Tomosyn, regulate how much neurotransmitter is available for release by controlling the number of synaptic vesicles, which store these small signaling molecules, that are available for release at synapses.
Littleton’s studies have also produced mechanistic insights into Huntington’s disease, epilepsy, autism and neuropathy. For instance, in 2005 a genetic screen yielded a mutation that caused seizures, modeling epilepsy. Follow-up research showed that the mutation caused non-neural “glial” cells to retain too much calcium. In 2019 the lab discovered that the excess calcium overactivated a protein called calcineurin, disrupting the glial cells’ removal of potassium ions from around neurons. That, in turn, meant neurons couldn’t get rid of excess potassium, leaving them electrically overexcited and prone to seizures. The research therefore provided a way to potentially target seizures without having to alter neurons directly.
“Diseases and proteins that affect neurons can cause devastating outcomes, but to study how that happens you can’t go into a human and manipulate them,” Littleton said. “Being able to manipulate the homologs of those in Drosophila and to use genetic approaches to put them into gene pathway networks then allows you to go back into mammals and begin to look at whether there are conserved molecular networks around the disease protein.”
Most Picower Institute labs work with mouse models because they, too, are small, easy to house and breed, and relatively simple compared to humans. But as mammals mice have brain cell types that match people closely—though not perfectly—and their brains are organized into regions (e.g. hippocampus) with functions (e.g. episodic memory) mimicking ours. Associate Professor Myriam Heiman’s studies of Parkinson’s disease, for instance, requires studying specific cell types in specific regions that mice and humans share.
“If we're interested in understanding the death of dopamine neurons in the midbrain, that's a relatively well conserved region between rodent and human brains,” Heiman said.
In her lab’s studies of neurodegenerative and psychiatric diseases, mice carrying human disease mutations can model how cells act and interact, circuits function and behaviors arise in health, disease and after potential treatments. She pairs such experiments and observations with analyses of postmortem human tissue.
Heiman’s use of mouse models has substantially advanced the field’s understanding of Huntington’s disease (HD), a devastating and so far untreatable neurodegenerative disease, by revealing new molecular mechanisms to target for therapies. In 2020 by performing an innovative genome-wide screen to delineate which genes promoted neural survival in HD model mice, Heiman identified the gene Nme1 and showed that increasing its expression in mice helped treat HD symptoms.
In another study that year, Heiman’s lab showed in both human brain tissue and mouse models that a potentially major and early mechanism of disease in HD is RNA leaking out of damaged mitochondria. She found it triggers an errant and harmful innate immune response in neurons especially vulnerable to the disease. In a 2022, study that mapped all the key cell types in the brain’s circulatory system both in healthy and HD humans and mice, the team saw the same sign of a misregulated immune response in the endothelial cells that help form the blood-brain barrier. The barrier protects the brain, so any harm to it could make disease worse.
Mice remain indispensable to Heiman’s efforts to identify new therapeutic strategies. But in studies where she gathers analogous data from both humans and mice, she compares them not only to see what’s the same, but also what’s different to ensure that both the potential and limits of mouse models are better understood. In the brain vasculature study, for instance, her team showed that while both mice and people exhibit “zonation,” or a difference in the cellular and molecular properties of arteries, veins and capillaries, the genes responsible in each species varied widely.
“Knowledge of these differences in zonation yields insight into differences in functionality between species,” Heiman said, including why some methods for delivering treatments through the bloodstream may work in mice but not humans.
In the lab’s newest paper, an analysis of what makes certain cell types especially vulnerable in ALS and Frontotemporal dementia, Heiman notes that key cells she and colleagues have pinpointed in humans aren’t present in mice.
New technologies including RNA sequencing cell by cell and the ability to engineer complex brain cell lab cultures from a human patient’s skin cells have improved what neuroscientists can learn from human samples (see our Winter 2019 edition), but these technologies can’t completely replace model organisms.
Complete behaving systems, simplified
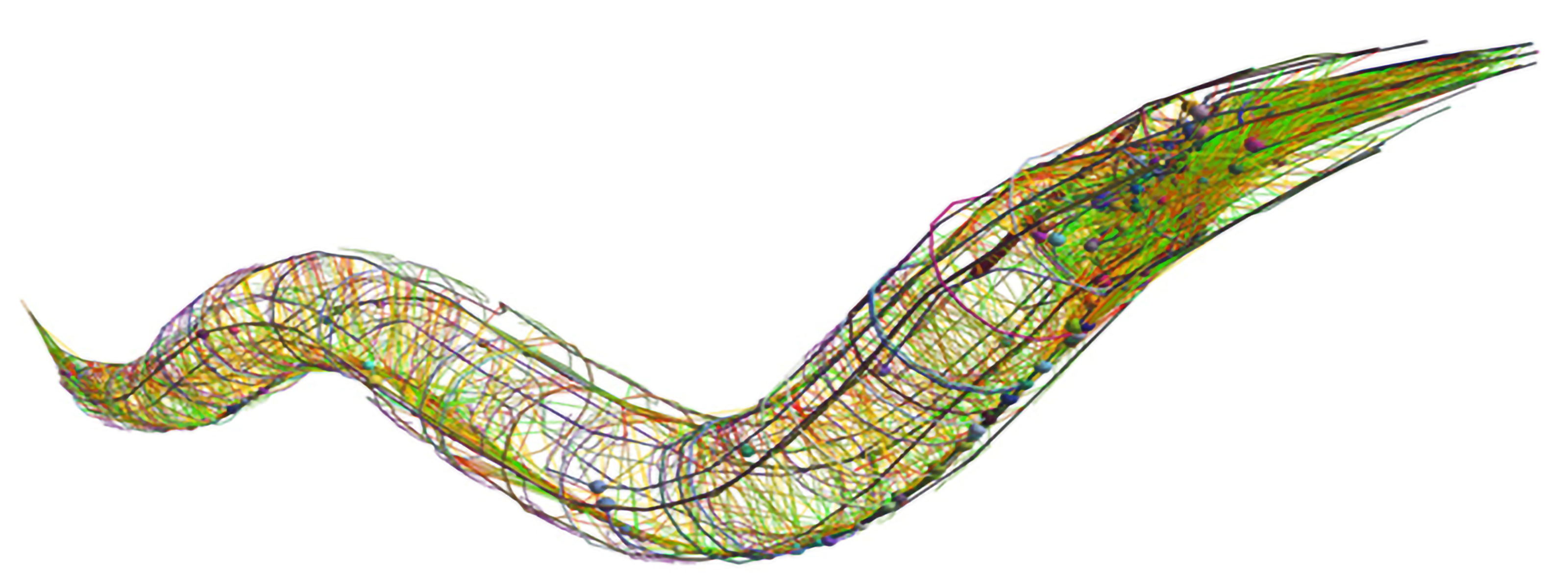
Only a live organism can demonstrate how cellular and molecular activity produce learning and memory, perception, consciousness, or behavior, or model how those are altered by disease. For neuroscientists who want to understand how these phenomena emerge from a whole nervous system, that question is incredibly complex to ask even in a mouse or fly. To make headway Associate Professor Steven Flavell studies how sustained but flexible behaviors emerge in the C. elegans roundworm, which has just 302 neurons. Tiny and transparent, C. elegans can be readily raised, imaged and genetically manipulated. All the connections among its neurons have been mapped out.
These advantages make C. elegans a tractable model of the brain-behavior connection, but in no way an easy or trivial one. Flavell has had to invent new technologies (e.g an imaging system capable of tracking every behavior while simultaneously mapping the activity of every neuron) and employ a host of cutting-edge methods (from gene editing to machine learning) to decode how the little worm uses the same neuromodulator chemicals we use to produce appropriate behaviors given its circumstances and internal needs.
For instance, in 2020 Flavell’s lab discerned how dopamine allows the worms to solve a problem all organisms face: coordinating multiple motor behaviors (in this case, cruising through a patch of food and laying eggs, as if methodically planting seeds in nutritive soil). And in a series of studies over a decade, Flavell’s lab has also built an unprecedentedly comprehensive understanding of how an animal brain employs serotonin, which in people is the most common target for psychiatric drugs. For instance, hungry worms stop and linger when they find a food patch and start eating. Flavell has shown this behavior depends on the neuron NSM emitting serotonin to inhibit neurons that govern locomotion. In the lab’s latest paper, published in the journal Cell, Flavell delineates how each of the worm’s six serotonin receptors responds to the chemical both individually and when expressed in combinations in neurons, provides a brain-wide mapping of which neurons harbor which combinations of the receptors, and uses this knowledge to produce a model that predicts how serotonin release changes brain-wide activity.
“C. elegans can’t tell us everything, but it has adapted ways to survive and thrive in its natural environment using behavioral strategies that have resemblances to humans. That provides an amazing opportunity for discovery,” Flavell said.
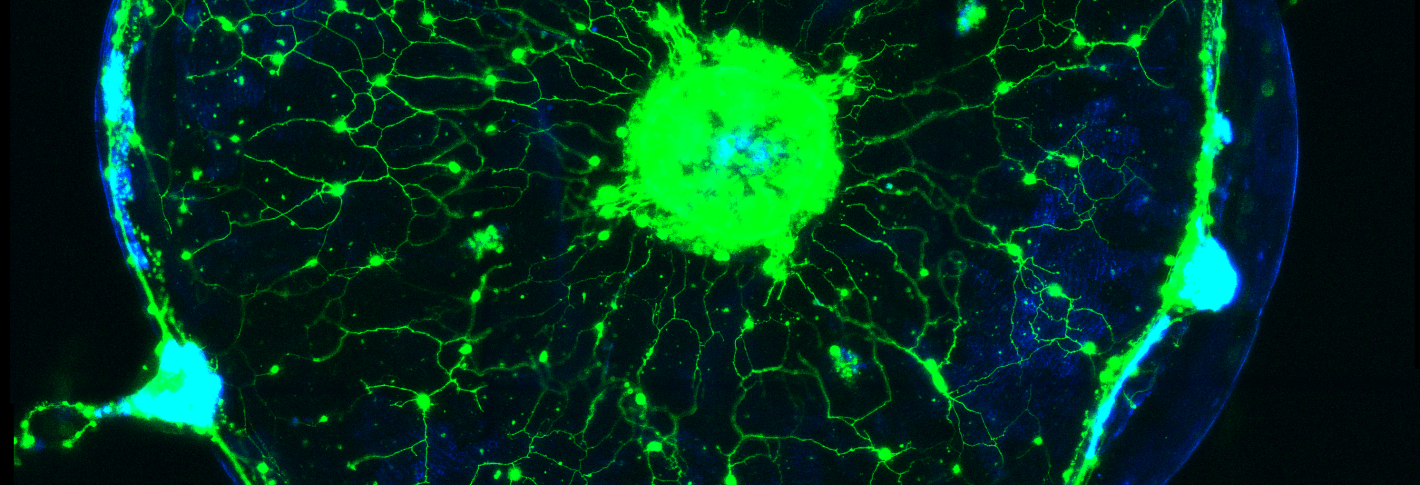
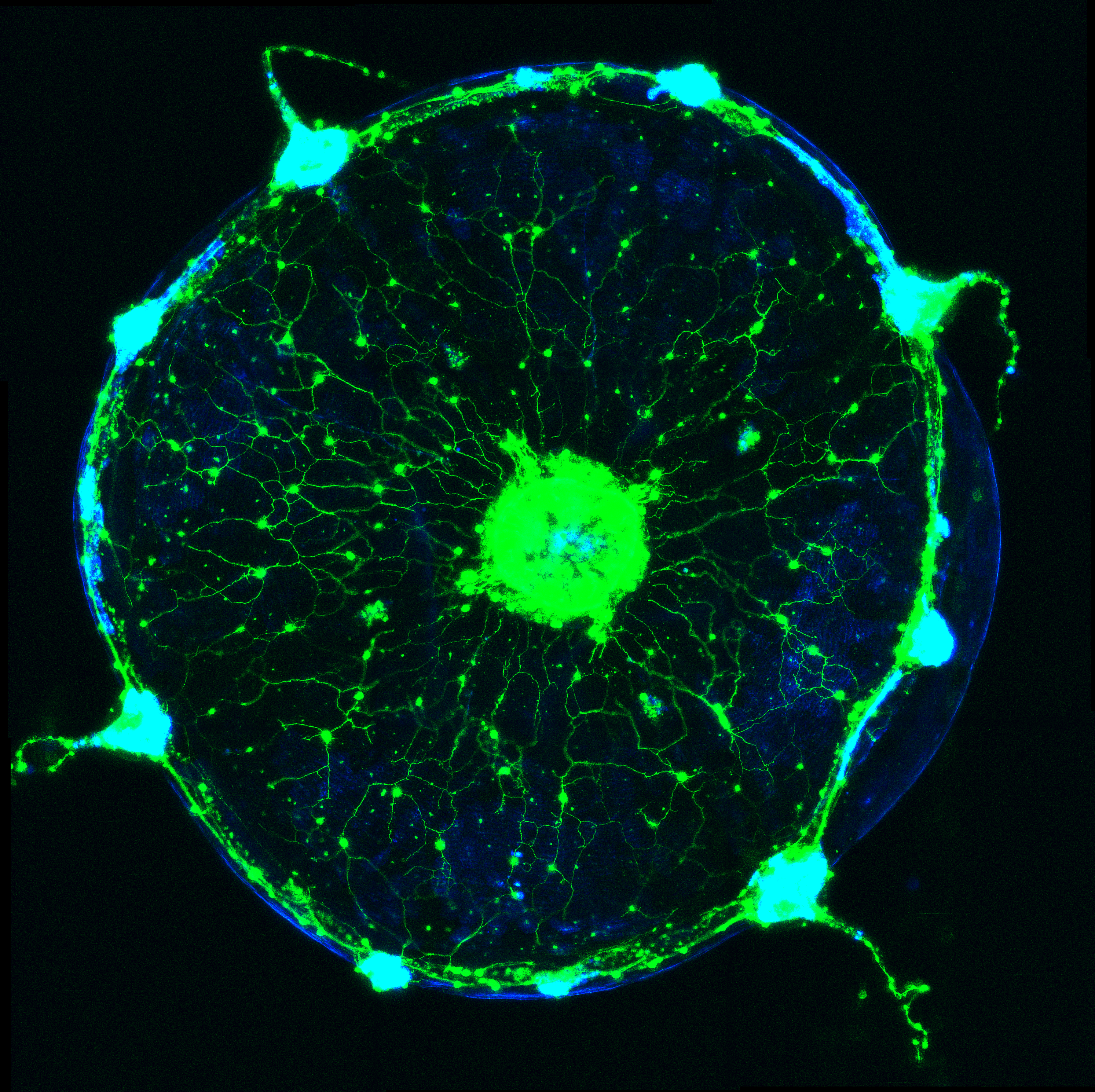
Weissbourd had two aims when he pioneered the use of C. hemisphaerica jellyfish as a model for systems neuroscience. One was that, like Flavell, he is interested in understanding fundamental, conserved principles of how behavior emerges from a whole nervous system. Though the jellyfish has no central brain, it produces complex behaviors appropriate for its context and its internal states, such as hunger. The jellyfish has several thousand neurons, is also transparent for easy imaging and has a uniquely advantageous life cycle in that its essentially immortal polyps can spawn exact genetic clones daily, Weissbourd said. That enables his lab to grow distinct genetic lines in perpetual abundance. His postdoctoral work focused on establishing methods for genetically engineering the jellyfish so that scientists can use modern research methods and experiments to interrogate how its neuronal activity produces behavior.
Having established this new model (and his new lab at MIT), Weissbourd has many open questions to pursue. Do they use the same neurotransmitters? How are their synapses structured? How do functions like perception and behavior emerge from these underlying properties and the organization of the jellyfish’s nervous system?
Diverse and different
If Weissbourd’s first aim is to understand the jellyfish’s similarities to other animals, the other is focused on understanding the value of how it is divergent. Jellyfish split off from the evolutionary branch that produced humans about 600 million years ago, not long after biologists believe the first nervous systems emerged. Fossils don’t preserve neurons, though, so the best way to make inferences about what features emerged in the very first nervous systems is to see what the most evolutionarily divergent organisms nevertheless have in common.
And the ways in which jellyfish outright differ can provide insight into alternative ways that nervous systems can maintain their health. Weissbourd’s postdoctoral work documented amazing degrees of regenerative capacity in the jellyfish nervous system. They are constantly adding new neurons so even when he wiped out a whole type of the cells (about 10 percent of its total neurons), the population restored rapidly (since then he’s found that the function those neurons enabled was only lost for about a week before the jellyfish rebuilt it).
In the cases when they are similar to us, as well as in the instances when they are very different, tiny model organisms can be enormously informative.