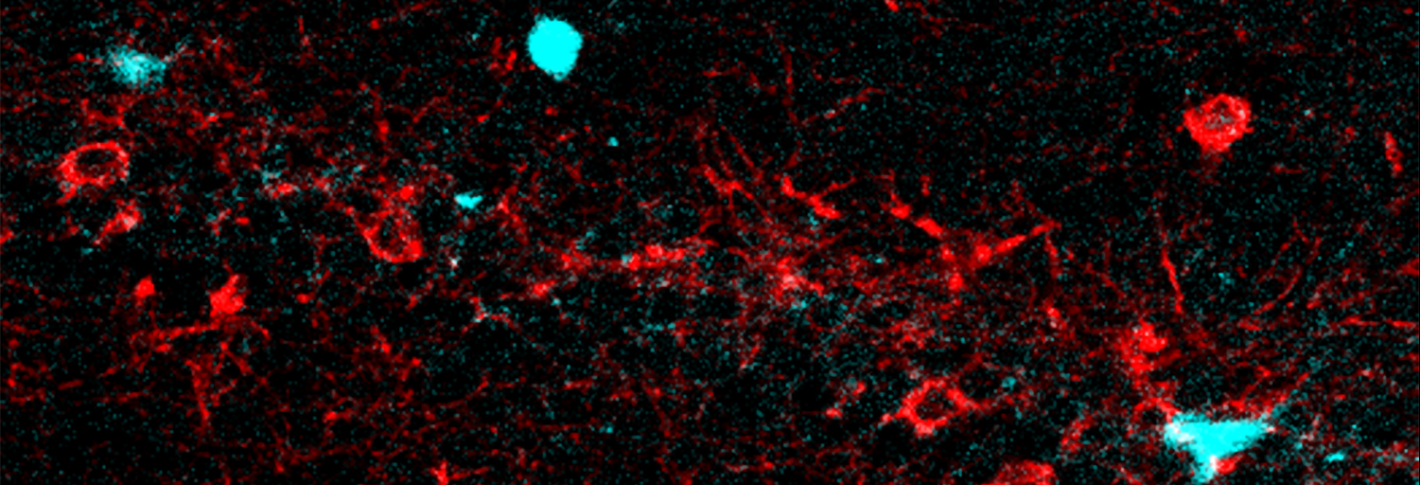
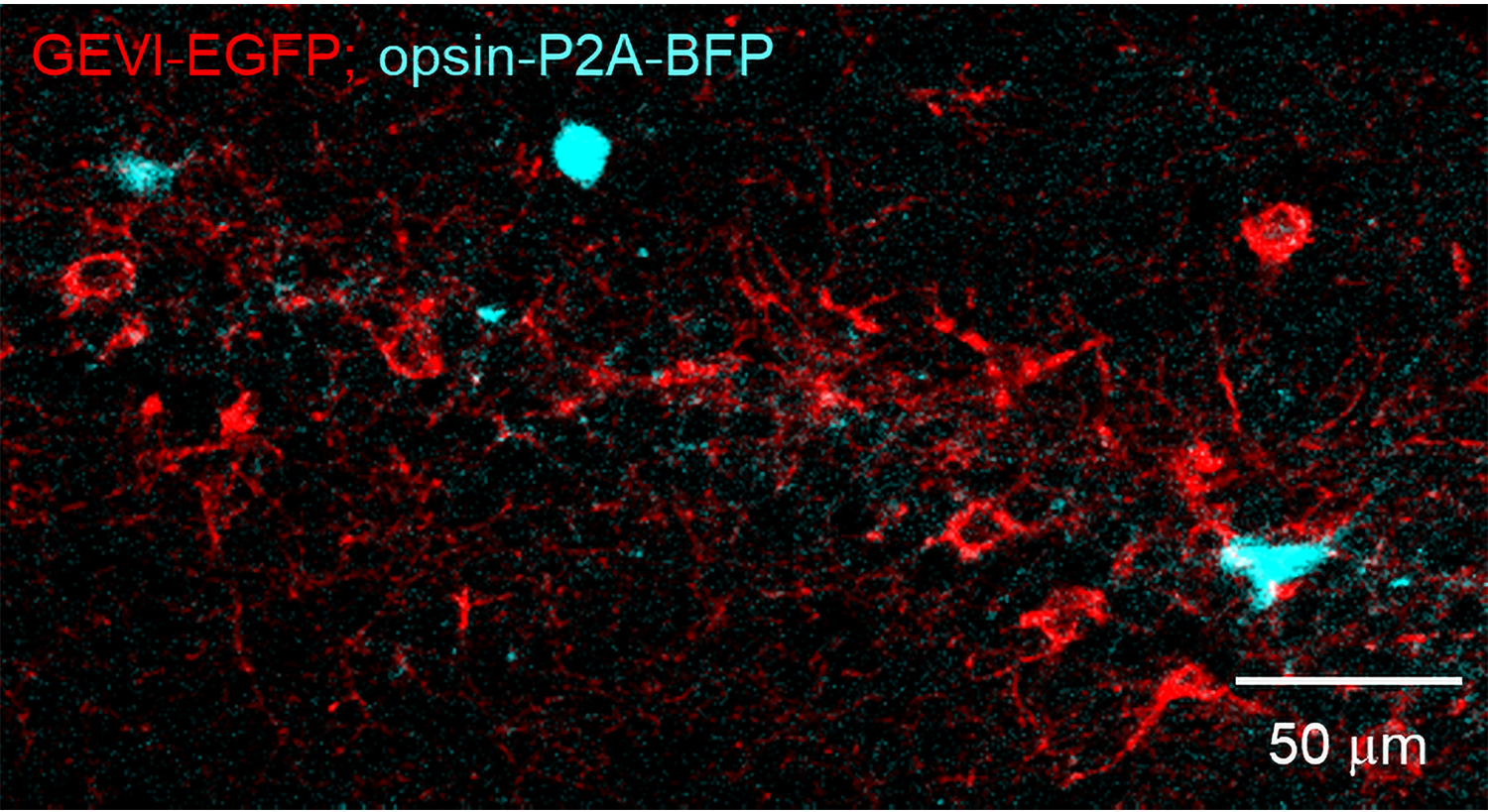
Knowing where you are is so important, the brain has special cells that dedicate themselves to the purpose. In a recent study in Science, a team of neuroscientists has demonstrated in live behaving animals a long-hypothesized mechanism that such “place cells” employ to refine that sense of location.
When place cells become activated at their target location they emit an endocannabinoid chemical signal to suppress the incoming circuit connections, called “synapses,” of a specific type of inhibitory neuron. If the scientists disrupted the endocannabinoid signaling during this process, place cells would lose their ability to refine their tuning to the location, making their sense of place less accurate.
Previous studies had only showed this particular way of altering neural circuit communication, or “synaptic plasticity,” in tissue slices rather than during actual spatial navigation behavior, said co-lead author Linlin Fan, assistant professor in The Picower Institute for Learning and Memory and the Department of Brain and Cognitive Sciences at MIT. Showing that this plasticity, called “depolarization-induced suppression of inhibition” (DSI) is key to refining memory of locations required multiple cutting-edge methods, she said. The emerging tools allowed the team, based at Stanford University at the time, to literally see and control the electrical activity of the circuit and to visualize the endocannabinoid signals.
“This study would have been very challenging using traditional methods,” Fan said. “We were in a very unique position to use optical methods to probe inhibitory synaptic transmission and plasticity.”
The new fundamental insight into the action of endocannabinoids in the brain, modeled in the mice, could help improve understanding in humans both of epilepsy and also the potential effects of marijuana use on learning and memory, the researchers said.
Optical observations
The researchers had mice run on a treadmill as key landmarks periodically passed them by. Meanwhile they labeled and observed two specific kinds of cells in the hippocampus region of the brain, which forms memories of places. One kind of cells were pyramid-shaped place cells, which become trained by circuit activity to represent a specific landmark. The other kind of cells were connected inhibitory cells, distinguished for expressing the protein CCK and for having receptors for endocannabinoids. The CCK cells typically reduce the electrical activity of the place cells via their shared synapses but scientists, using dissected tissue, had noticed that when place cells were artificially electrically stimulated, they could tamp down inhibitory input via endocannabinoid-mediated DSI.
In the new study Fan and co-lead and corresponding author Barna Dudok, now at Baylor College of Medicine, set out to determine whether this suppression of inhibition happens in live behavior, how endocannabinoid signaling plays its role, and what it means for learning and memory.
In one set of experiments, the researchers used a sensor molecule called GRAB that co-author Yulong Li at Peking University invented and Dudok helped to characterize. GRAB glows in the presence of endocannabinoids. Simultaneously they engineered place cells to glow when they built up calcium ions, a proxy for electrical activity. They saw that when mice reached a location that made a place cell become active, the cell would release endocannabinoids. Those endocannabinoids would then show up at the synapses that inhibitory cells made with the place cells.
In another set of experiments the team sought to examine what impact this “retrograde” endocannabinoid signaling had on the inhibition that CCK cells effect across those synapses with place cells. They used two technologies that Fan has pioneered using in combination, for instance in a study in Cell last year that demonstrated the excitatory inputs that help place cells attune to locations to begin with. With “optogenetics” they took control of the activity of the CCK cells. Then, using a genetically encoded voltage indicator, a protein that glows to quickly and precisely indicate electrical activity, they watched the membrane voltage of connected pyramid-shaped, or “pyramidal,” cells. They saw that when the pyramidal cells experienced complex spikes of circuit activity (for instance, because the mouse had reached the tuned location), then generating a signal from the CCK cells could not impart much inhibition. If the pyramidal cells didn’t experience such spikes of activity, then generating an inhibitory signal from CCK cells did inhibit the pyramidal cells.
“We saw suppression of this inhibitory input from the CCK cells to the pyramidal cells when the pyramidal cells displayed complex spikes before the inhibition,” Fan said.
Finally, the researchers genetically knocked out endocannabinoid receptors in the inhibitory neurons to see what would happen if the signaling couldn’t occur. When they did, they found that the place cells fired less precisely and reliably at their attuned landmarks than if endocannabinoid signaling were not knocked out.
Mouse evidence; human significance
Fan said the study results raise new questions about how cannabis use could affect this endocannabinoid-dependent learning and memory process.
“The active ingredient in cannabis can ‘hijack’ this endocannabinoid pathway,” Fan said.
She plans to probe the mechanism further by using optogenetics to induce the complex spikes that trigger DSI. Such experiments cold provide insight into the timing required for it to happen.
And because previous research has indicated that epileptic seizures trigger endocannabinoid release, the new study may help inform whether and how cannabis-related drugs could help treat epilepsy.
In addition to Fan, Dudok and Li, the paper’s other authors are Jordan S. Farrell, Shreya Malhotra, Jesslyn Homidan, Doo Kyung Kim, Celestine Wenardy, Charu Ramakrishnan, Karl Deisseroth, and Ivan Soltesz.