“Our goal is to target a protein that regulates gene expression in microglia by developing a novel, small molecule compound to the point where it will be ready for phase I clinical trials,” said Li-Huei Tsai, director of The Picower Institute for Learning and Memory at MIT and primary investigator on the grant, which will support the effort with up to $8 million over the next five years. “Success will provide a new therapeutic avenue that is desperately needed for this devastating neurological disease.”
Tsai’s lab will collaborate with those of NDC director William J. Ray, director of the drug discovery program at The University of Texas MD Anderson Cancer Center, and fellow NDC member Alison Goate, director of Ronald M. Loeb Center on Alzheimer’s disease at Icahn School of Medicine at Mount Sinai.
In a paper in 2015 Tsai and MIT Computer Science Professor Manolis Kellis identified the protein PU.1 as a transcription factor responsible for regulating the expression of genes in microglia that were misregulated in Alzheimer’s model mice and human patients. In 2017 Goate led a study showing that people with a genetic variation that reduced expression of the gene that encodes PU.1 had a significantly lower risk of developing Alzheimer’s and did so at later age.
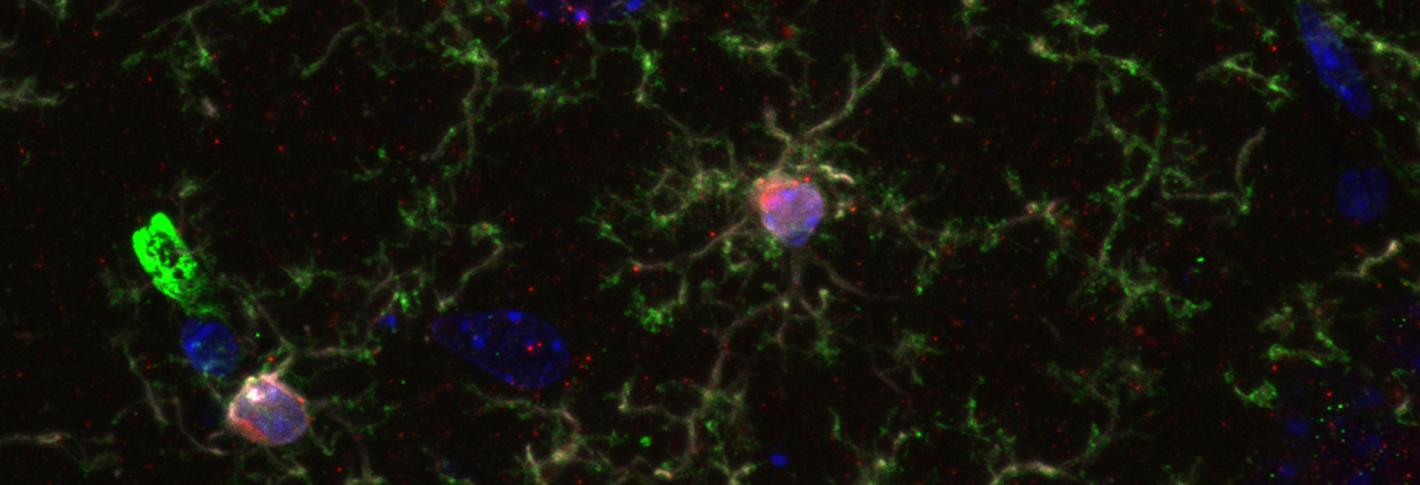
Above: Microglia have emerged as important cells of interest in Alzheimer's disease. Credit Leyla Akay
Given this data, the NDC collaborators, including Ray’s drug development team, have been pursuing the hypothesis that finding a drug that can inhibit PU.1 activity can delay the onset of Alzheimer’s. Their early efforts were funded by the Robert A. and Renee E. Belfer Family Foundation. After an extensive screening led by Tsai lab postdoc William Ralvenius, they have identified a compound with strong and specific effects in reducing PU.1 activity and modulating the expression of the same genes that PU.1 is responsible for regulating.
“We started this project with more than 58,000 molecules and identified a handful of drug-like compounds after multiple rounds of validation,” Ralvenius said. “Our top candidate shows promising therapeutic potential in both mouse and human microglia. We are now refining its structure to improve its property for subsequent in vivo experiments and clinical applications.”
In initial experiments the collaboration has found that reducing PU.1 activity improves the ability of cultured human and mouse microglia cells to combat Alzheimer’s disease pathology.
With the new grant the team will now work to develop versions of the compound that are optimized for eventual therapeutic testing. In the first few years of the project they will develop versions of the compound for maximal efficacy and safety in two different mouse models of Alzheimer’s and in human cell cultures. They will also conduct studies to identify the specific molecular targets and mechanisms by which PU.1 inhibiting compounds work. Once targets are known and a compound has been sufficiently optimized for safety and efficacy in the lab, the team will refine it further for eventual human testing including determining the likely optimal dose and rooting out off-target effects.
Ultimately the team’s hope is that the compound will essentially mimic the effect of the Alzheimer’s protective genetic variation Goate identified, thereby delaying the onset of Alzheimer’s in patients.
“Even a few years delay in the age of Alzheimer’s onset would provide tremendous benefit to millions of people, their caregivers and families and healthcare systems worldwide,” the team’s grant proposal states.