Director, The Picower Institute for Learning and Memory; Lead Investigator, Aging Brain Initiative; Picower Professor of Neuroscience, Department of Brain and Cognitive Sciences; Associate Director, Paul F. Glenn Center for Science and Aging Research; Associate Member, Broad Institute
Today, an estimated 55 million people worldwide suffer from Alzheimer’s disease or some other form of dementia. That number is expected to double every 20 years as life expectancy rises and populations age.
Currently, there are no effective therapies and the economic burden of Alzheimer’s disease is unsustainable; in the United States alone, care for Alzheimer’s patients is estimated to total $1.1 trillion by 2050.
"Our MIT scientists are opening the doors to an entirely new direction of brain research. It is a priority for me to support their efforts,” says ABI co-founder Michael Sipser, MIT’s dean of science from 2014 to 2020 and Donner Professor of Mathematics. "The tremendous need to address the burdens of the aging brain — memory loss, cognitive decline, and dementia — is what gave rise to an Institute-wide call to action at MIT."
About the Aging Brain Initiative
"la Caixa" Fellowships
Learn more about a special set of fellowships for postdoctoral researchers intersted in studying the aging brain.
This interdisciplinary research effort pulls together faculty expertise, knowledge, and technical resources from across MIT to solve the mysteries of the aging brain. It spans neuroscience, fundamental biology and genetics, investigative medicine, engineering and computer science, economics, chemistry, urban planning, and artificial intelligence to enable a comprehensive systems approach. What's the ultimate mission? To deliver the basic research that makes possible new tools to address the challenges of brain aging and create a better future for millions.
Annual newsletter
Our annual newsletter provides a digest of the findings and progress ABI-affiliated labs are achieving in their research.
Full list of faculty
Click here to see and contact the full list of the faculty involved.
Y. Eva Tan Professor in Neurotechnology, Departments of Biological Engineering and Brain and Cognitive Sciences ; Associate Professor of Media Arts and Sciences, MIT Media Lab; Member, McGovern Institute for Brain Research

Edward Hood Taplin Professor of Medical Engineering and Computational Neuroscience; Warren M. Zapol Professor of Anesthesia, Harvard Medical School and Massachusetts General Hospital; Director, Harvard-MIT Health Science and Technology Program; Associate Director, Institute for Medical Engineering and Science; Investigator, The Picower Institute for Learning and Memory

Novartis Professor of Biology, Department of Biology; Director, Paul F. Glenn Center for Science and Aging Research; Affiliate, Koch Institute for Integrative Cancer Research

Nobel Laureate; David H. Koch Professor, Department of Biology; Member, McGovern Institute for Brain Research; Member, Koch Institute for Integrative Cancer Research; Investigator, Howard Hughes Medical Institute

Professor, Department of Electrical Engineering and Computer Science; Principal Investigator, Computer Science and Artificial Intelligence Lab; Institute Member, Broad Institute

Former Dean, School of Science
Donner Professor of Mathematics
Nobel Laureate; Picower Professor of Biology and Neuroscience, Departments of Biology and Brain and Cognitive Sciences; Investigator, The Picower Institute for Learning and Memory; Director, RIKEN-MIT Center for Neural Circuit Genetics; Director, RIKEN-Brain Science Institute
The Frontier of Brain Science
Breakthrough Discovery
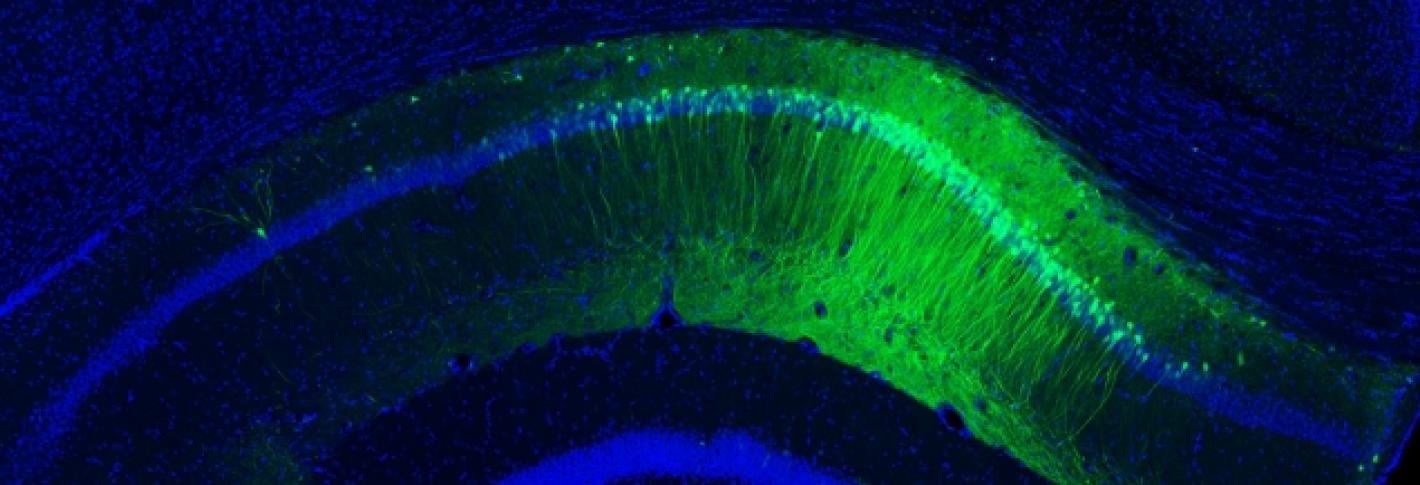
In the brain, large groups of interconnected neurons can fire together in a regular, synchronized manner to produce long-range oscillations, or brain waves. One particular set of these rhythmic patterns, called gamma waves, is known to be involved in higher-order brain functions such as attention, sensory perception, and memory—the same cognitive functions affected by aging. In Alzheimer’s disease, the brain no longer produces gamma wave oscillations, even before cognitive symptoms can be detected. Li-Huei Tsai, Picower Professor and Director of the Picower Institute for Learning and Memory, wondered if this brain circuit failure could be causing the disease progression, and if functionality could be restored. She discovered that indeed, it may.
In collaboration with MIT professors Edward Boyden ’99, MNG ’99 and Emery Brown, Tsai found that they could stimulate gamma waves in the brains of mice with Alzheimer’s disease by exposing them to lights flashed on and off at a particular frequency, about 40 cycles per second. In effect, the light signals activated the brain and trained neurons to fire at gamma frequencies.
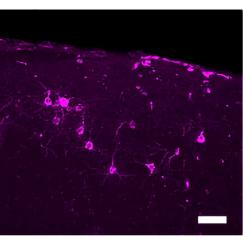
Other results produced by this light therapy were just as stunning. First, brain levels of beta amyloid, the main component of the sticky plaques built up in the brains of Alzheimer’s patients, were almost cut in half with an hour of exposure to the light. Repeated exposures—an hour a day for a week—produced reductions in amyloid levels and in the sticky amyloid plaques. In addition, the flickering lights and the resultant gamma waves reactivated sluggish microglia, the brain’s immune cells. The microglia became larger and started scavenging amyloid peptides.
Restoring gamma waves also appeared to help keep brain cells from dying—preventing brain shrinkage. Moreover, the brain’s vasculature started to expand, which in addition to reducing amyloid production may help also clear amyloid from the brain.
Most importantly, however, the mice treated with the flickering lights could learn again, and cognitive tests showed that their memory improved. In mice, it seems this therapy makes the brain healthier and able to work towards treating its own Alzheimer’s disease.
The question now is: can this therapy have the same results in humans suffering from Alzheimer’s? If it can, this would be the first noninvasive and effective treatment available.
Patients would be able to administer it themselves—perhaps by wearing a special type of headset that emits the light, or sitting in a room with a device that flashes at 40 hertz for a period every day. The MIT team is already preparing human studies to test such devices. They are also looking at whether other sensory modalities, such as sound or smell, can produce the same effects.
"It’s possible that gamma wave therapy could benefit other diseases besides Alzheimer’s or even enhance normal memory,” Boyden SAYS. “Perhaps gamma stimulation could be embedded in our music, our TV shows. The possibilities are amazing."
A Systems Approach
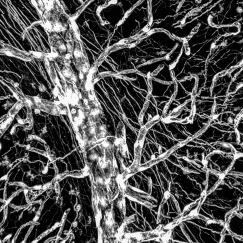
Over the past five years, impressive advances in technologies have enabled us to more fully explore the brain and its complex circuitry of neurons. We now have better ways to see deep inside the brain and trace neural networks in unprecedented detail along with methods that allow us to stimulate specific neurons using laser light. These new tools have enabled researchers to more precisely study the neural circuits and brain activity patterns that encode our memories, translate our movements, and predict our decisions.
Restoring Memories
MIT’s Picower Professor Susumu Tonegawa, who studies memory, discovered how to identify the specific brain cells that encode a particular memory. He also developed a way to stimulate specific brain cells at specific times by inducing a light-sensitive protein within them and then pulsing them with laser light. In experiments with mice in the early stages of Alzheimer’s disease, Tonegawa’s team was able to use these techniques on memory cells to trigger them to recover short-term memories, such as a fear of a particular place, which the mice had forgotten. This research suggests strongly that memory loss in Alzheimer’s disease is actually caused by a loss of access to memories and not the loss of the memory itself. Even when affected by dementia, Tonegawa found, the brain still has the ability to learn and to store a memory.
Tonegawa and his team also found that strengthening the synaptic connections of those memory cells enabled longer-term restoration of the lost memories. Knowing that memory reactivation is possible, MIT researchers such as Boyden and Tsai are exploring noninvasive ways, such as using electricity or magnets transcranially to target deep and specific locations in the brain, to stimulate memory cells in human patients.
Improving Cognition
Where in the brain does Alzheimer’s disease begin? Using the new-tissue clearing methods to examine neural circuits in unprecedented detail, Tsai in collaboration with assistant professor Kwanghun Chung have found that the first signs of degradation occur in deep brain regions that are involved in brain-wide memory processing. These actions may then trigger a cascade that affects the circuits that store short-term memories of everyday events. Tsai’s team is now tracing how these brain networks decline. Because memory circuit malfunction is likely to be a primary cause of cognitive decline, brain stimulation therapies—including brain wave stimulation—may be able to restore healthy network patterns and improve memory and cognitive capacity, while also potentially modifying the course of the disease.
Novel drug therapies may be another approach to restoring memories. In mouse experiments, Tsai’s team used molecules that inhibit enzymes known to regulate gene expression involved in forming memories. These drugs can turn on genes that had otherwise been blocked, and are able to bring back “lost” memories in mice with Alzheimer’s disease. The researchers are now exploring whether this approach will be effective in treating Alzheimer’s disease or other degenerative diseases in humans.
Biomarkers of Unhealthy Aging
Neuroscientists face a critical knowledge gap—they don’t know why some people age well and others do not, or how their brains differ in their aging process. One promising strategy to bridge this gap is to map what is known as the human epigenome—in effect, to map which genes are turned on in which cells in all parts of the body. This effort is part of a national project that MIT and professor Manolis Kellis ’99, MNG ’99, PhD ’03, are leading, and which will collect massive amounts of data from many different individuals. By applying MIT’s strength in big data strategies and artificial intelligence “deep-learning” techniques to this data, Kellis, in collaboration with Novartis Professor of Biology Leonard Guarente ’74 and Tsai hope to identify key biomarkers for healthy and unhealthy aging. Such biomarkers would enable both early diagnosis and ways to track the progression of disease or improvements from therapies. Already, Kellis and Tsai have found a biomarker in the regulatory area of the brain’s immune genes that is a major risk factor for Alzheimer’s disease.
Measuring Brainwaves for Early Diagnosis
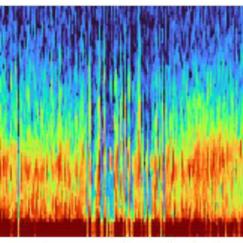
Anesthesia affects the brain differently as people age. MIT’s Emery Brown, the Edward Hood Taplin Professor of Medical Engineering and of Computational Neuroscience—who is both a computational neuroscientist and an anesthesiologist—uses EEG, a technology that uses electrodes to record brain waves, to show that patterns and strength of brain waves change with age. He has characterized differences in brain waves in patients under anesthesia between the ages of 18 and 90 years, and is using these data to create a brain-age calculator. The technique has the potential to analyze differences in patients with and without Alzheimer’s disease, or to gauge how well an individual’s brain is aging, and thereby help identify brains whose wave patterns suggest they may be at a higher risk of developing Alzheimer’s disease, even well before the cognitive decline and other behavioral symptoms appear.
Bringing New Tools and Therapies to Market
Research is powerful. But discoveries only benefit people once the products from discovery are approved by regulatory agencies and become commercially available. Because MIT anchors what is arguably the biotech capital of the world—an enormous concentration of biotech companies and research centers—it can rapidly translate new discoveries into practical applications ready for the marketplace. MIT’s location also means that the Aging Brain Initiative can collaborate with—and leverage skills and talent from—a wide array of partners. As a result, the members of the Aging Brain Initiative are confident that their research will go a long way toward halting or significantly slowing the progression of Alzheimer’s disease and other dementias and improve cognitive outcomes in those afflicted with these conditions.
To Make a TrANSFORMATIONAL Gift
OR FOR MORE INFORMATION , Contact:
Asha Bhakar, PhD
Director of Development
abhakar@mit.edu
(617) 258-0759
Study finds tracking brain waves could reduce post-op complications
Atlas of human brain blood vessels highlights changes in Alzheimer’s disease
40 Hz vibrations reduce Alzheimer’s pathology, symptoms in mouse models
Neuroscientists identify cells especially vulnerable to Alzheimer’s
A new peptide may hold potential as an Alzheimer’s treatment
How Huntington’s disease affects different neurons
Pagination
.
Investing in Talent — The Aging Brain Initiative Fund
The only way to decode the mysteries of the brain and to find a cure or better treatments for the dementias of aging—and to build on the momentum already created by the Aging Brain Initiative—is to support the innovation pipeline: the faculty, students, and other scientists engaged in fundamental brain aging research, and the tools and facilities that enable their work.
What does innovation cost? $1M to endow a research fellowship that supports next-generation scientists; $2M–$4M to seed ambitious new research projects; $5M–$20M to support an intensive flagship research program focused on a specific problem; $25M–$50M to enable a broad gauge, MIT-wide research effort for 5 to 8 years; $100M–$150M to establish an endowed named research center to support aging brain research in perpetuity.
Specific examples include:
Documenting the Benefits and Biomarkers of Gamma Wave Therapy in Alzheimer’s Disease.
A gift of $5M would support 5 years of advanced preclinical/early clinical research to measure gamma stimulation benefits, develop 40-hertz devices, and discover biomarkers to guide clinical trials and medical practice.
Creating Noninvasive Multimedia Brain Wave Therapies for Human Use.
A gift of $5M would support 5 years of advanced preclinical/early clinical research exploring gamma wave stimulation through sound and how to embed the therapy in multimedia experiences.
Pushing the Innovation Envelope.
A gift of $5M could enable breakthrough research in using artificial intelligence to identify aging biomarkers, developing therapies that target brain circuits or personalized approaches to treatment, or exploring the secrets of healthy aging.
Our target goal of $250M would enable the Aging Brain Initiative to fully fund the array of promising research and therapy development opportunities. Partnership opportunities are available at multiple levels.
The Aging Brain Initiative Fund
Promote interdisciplinary research that is striving to identify and model the biomarkers of brain aging, develop circuit-specific therapeutics and personalized treatments, and demonstrate how to promote brain resilience.
To Make a TrANSFORMATIONAL Gift
OR FOR MORE INFORMATION , Contact:
Asha Bhakar, PhD
Director of Development
abhakar@mit.edu
(617) 258-0759