To understand the full relationship between brain activity and behavior, scientists have needed a way to map this relationship for all of the neurons across a whole brain—a so far insurmountable challenge. But after inventing new technologies and methods for the purpose, a team of scientists in The Picower Institute for Learning and Memory at MIT has produced a rigorous accounting of the neurons in the tractably tiny brain of a humble C. elegans worm, mapping out how its brain cells encode almost all of its essential behaviors, such as movement and feeding.
In the journal Cell, the team presents new brain-wide recordings and a mathematical model that accurately predicts the versatile ways that neurons represent the worm’s behaviors. Applying that model specifically to each cell, the team produced an atlas of how most cells, and the circuits they take part in, encode the animal’s actions. The atlas therefore reveals the underlying “logic” of how the worm’s brain produces a sophisticated and flexible repertoire of behaviors, even as its environmental circumstances change.
“This study provides a global map of how the animal’s nervous system is organized to control behavior,” said senior author Steven Flavell, Associate Professor in MIT’s Department of Brain and Cognitive Sciences. “It shows how the many defined nodes that make up the animal’s nervous system encode precise behavioral features, and how this depends on factors like the animal’s recent experience and current state.”
Graduate students Jungsoo Kim and Adam Atanas, who each earned their PhDs this spring for the research, are the study’s co-lead authors. They’ve also made all their data, and the findings of their model and atlas, freely available to fellow researchers at a website called the WormWideWeb.
Microscopes to models
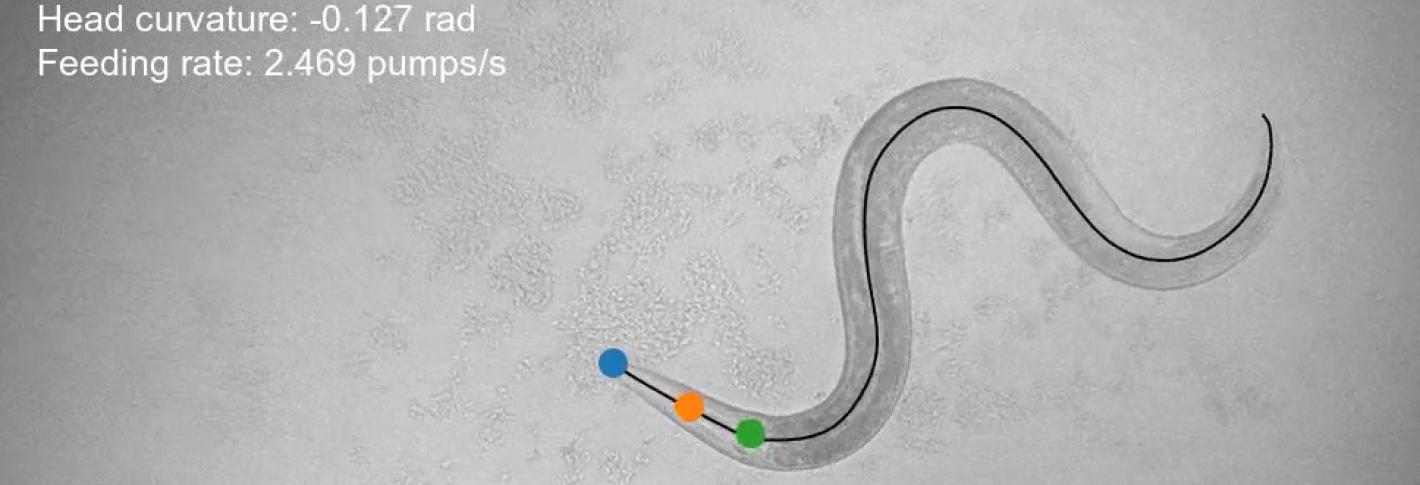
To make the measurements needed to develop their model, Flavell’s lab invented a new microscope and software system that automatically tracks almost all behaviors of the worm (movement, feeding, sleeping, egg-laying, etc.) and the activity of every neuron in its head (cells are engineered to flash when calcium ions build up). Reliably distinguishing and tracking separate neurons as the worm wriggles around and bends required writing custom software, utilizing the latest tools from machine learning. It proved to be 99.7 percent accurate in sampling individual neuron’s activities with greatly improved signal-to-noise compared to previous systems, the scientists report.
The team used the system to record simultaneous behavior and neural data from more than 60 worms as they roved about their dishes, doing whatever they wanted.
Data analysis revealed three novel observations about neural activity in the worm: Neurons track behavior not only of the present moment but also the recent past; they tune their encoding of behaviors, such as motion, based on a surprising variety of factors; and many neurons simultaneously encode multiple behaviors.
For example, while the behavior of wriggling around one’s little laboratory dish might seem like a very simple act, neurons represented factors such as speed, steering, and whether the worm is eating or not. In some cases they represented the animal’s motion spanning back in time by about a minute. By encoding recent, rather than just current motion, these neurons could help the worm compute how its past actions influenced its current outcome. Many neurons also combined behavioral information to execute more complex maneuvers. Much like a human driver must remember to steer the car in the opposite way when going in reverse versus going forward, certain neurons in the worm’s brain integrated the animal’s direction of motion and steering direction.
By carefully analyzing these kinds of patterns of how neural activity correlated with behaviors the scientists developed the C. elegans Probabilistic Neural Encoding Model. The model, encapsulated in a single equation, accounts for how each neuron represents various factors to accurately predict whether and how the neural activity reflects behavior. Nearly 60 percent of the neurons in the worm’s head indeed accounted for at least one behavior.
In fitting the model, the research team used a probabilistic modeling approach that allowed them to understand how certain they were about each fit model parameter, an approach pioneered by co-author Vikash Mansinghka, a principal research scientist who leads MIT’s Probabilistic Computing Project.
Making an atlas
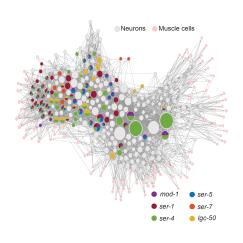
In creating a model that could quantify and predict how any brain cell would represent behavior, the team initially gathered data from neurons without tracking the cells’ specific identities. But a key goal of studying the worms is to understand how each cell and circuit contributes to behavior. So to apply the model’s capability to each of the worm’s specific neurons, which have all been previously mapped out, the team’s next step was to relate neural activity and behavior for each cell on the map. Doing that required labeling each neuron with a unique color so that its activity could be associated with its identity. The team did this in dozens of freely-moving animals, which provided them with information of how almost all of the defined neurons in the worm’s head related to the animal’s behavior.
The atlas resulting from this work revealed many insights, more fully mapping out the neural circuits that control each of the animal’s behaviors. These new findings will enable a more holistic understanding of how these behaviors are controlled, Flavell said.
“It allowed us to complete the circuits,” he said. “Our hope is that as our colleagues study aspects of neural circuit function, they can refer to this atlas to obtain a fairly complete view of the key neurons involved.”
Built for flexibility
Another major outcome of the team’s work was the finding that while most neurons always obeyed the predictions of the model, a smaller set of neurons in the worm’s brain—about 30 percent of those that encode behavior—was able to flexibly remap their behavior encoding, essentially taking on new jobs. The neurons in this group were reliably similar across animals, and were well connected with one another in the worm’s synaptic wiring diagram.
Theoretically these remapping events could occur for any number of reasons, so the team ran further experiments to see if they could cause neurons to remap. As the worms wriggled around their dishes, the researchers applied a quick laser zap that heated the agar around the worm’s head. The heat was harmless but enough to annoy the worms for a while, inducing a change in the animal’s behavior state that lasted for minutes. From these recordings the team was able to see that many neurons remapped their behavioral encoding right as animals switched behavioral states.
“Behavioral information is richly expressed across the brain in many different forms – with distinct tunings, timescales, and levels of flexibility – that map onto the defined neuron classes of the C. elegans connectome,” the authors wrote.
In addition to Atanas, Kim, Mansinghka, and Flavell, the paper’s other authors are Ziyu Wang, Eric Bueno, McCoy Becker, Di Kang, Jungyeon Park, Talya Kramer, Flossie Wan, Saba Baskoylu, Ugur Dag, Elpiniki Kalogeropoulou, Matthew Gomes, Cassi Estrem, and Netta Cohen.
Funding sources for the research include the National Institutes of Health, the National Science Foundation, The McKnight Foundation, The Alfred P. Sloan Foundation, The Picower Institute for Learning and Memory and The JPB Foundation.