The things we do for love. The mistakes we make when we’re tired or rushed. The angry words we wish we could take back. The times we jump for joy.
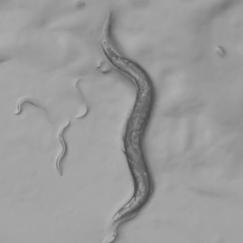
The episodes when our internal emotions and motivations affect our behavior provide some of our strongest and most personal experiences with our selves. But as intensely felt as these times and underlying states can be, they have been difficult for neuroscientists to define and explain. Related disorders such as depression can therefore be harder to treat. As a newly named HHMI Investigator, Associate Professor Steve Flavell directs a research program in a much simpler organism, the roundworm C. elegans, that he hopes will prove foundational to building an understanding of how internal states arise and influence behavior in nervous systems in general.
“I think that it should be possible to define the basis of internal states in C. elegans in concrete terms ,” Flavell said. “If we can build a thread of understanding from the molecular architecture of neuromodulatory systems, to changes in brain-wide activity, to state-dependent changes in behavior, then I think we’ll be in a much better place as a field to think about the basis of brain states in more complex animals.”
The long-term impact would not only be a better understanding of the link between brain activity and behavior, but also improved interventions for psychiatric disorders. Many psychiatric medicines target neuromodulatory brain chemicals, such as serotonin, that lie at the heart of the dynamics Flavell is investigating.
Indeed, these goals have guided Flavell’s lab in The Picower Institute for Learning and Memory and the Department of Brain and Cognitive Sciences at MIT ever since he established it in 2016, but now with the HHMI’s long-term, flexible support he can address the full set of scientific challenges necessary to answer these questions. HHMI’s “people, not projects” approach in supporting the investigators it selects provides generous, renewable funding that provides the flexibility to let investigators go where ever their scientific questions take them.
“This allows us to think about these scientific problems in a manner that embraces their full complexity,” said Flavell, who is one of four MIT faculty members and 26 scientists around the country to be named HHMI Investigators this year.
A multidisciplinary research program
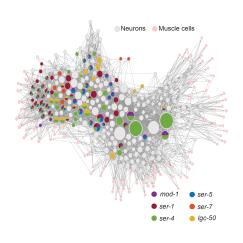
C. elegans is an animal that is simple enough to be studied from the molecular to whole-brain scale, but deceptively complex nonetheless. Though the entire connectome, or wiring diagram, among its 302 neurons has been fully mapped and its genes are readily manipulable in lab experiments, the worm nevertheless displays a remarkable array of flexible behaviors that often employ the same molecular machinery found in humans, including neuromodulatory chemicals such as serotonin and dopamine. In all, the worm has more than100 different neuromodulators that might enable its internal states to change how it behaves.
In recent years, Flavell’s lab has published studies showing how specific neural circuits, employing neuromodulators, integrate inputs such as sensory information and internal states to produce flexible behaviors. In two papers last year, his lab and collaborators blended techniques ranging from genetic and molecular manipulations, to imaging of behavior and brain-wide neural activity, to computational modeling to produce major brain-wide insights. In one study, the team built a model that can predict how a majority of the worm’s neurons encode and represent its behaviors, and found that about 30 percent of the worm’s neurons remain flexible to account for different behavioral situations. In another paper the scientists thoroughly mapped the worm’s six distinct serotonin receptors (humans have 14) across each neuron in its brain, and showed how serotonin release acts on each of them to affect feeding and locomotion.
Framework for the future
One of the main new thrusts of the lab’s work, Flavell said, will be to build on those achievements by constructing a new generation of computational models that predict how every neuron responds to a wider range of internal states in the worm.
Another new area of work will delve deeper into how neuromodulators affect the animal’s internal states, ranging from studies of how these chemicals are released and diffuse to reach their targets and studies of how they act in combination, Flavell said.
A third major initiative in the lab will tackle the question of how specific features of the connectome – aspects of nervous system wiring – control internal states.
A recognition of the whole lab
The HHMI award’s support doesn’t just open up these new opportunities for Flavell, but for his whole lab. As a team of postdocs, graduate students, technicians and other trainees, Flavell said, his lab will now have greater flexibility to consider the biggest questions and implications of their results and to take whatever approaches are needed to investigate them.
In that spirit, as Flavell reflected on being named an HHMI Investigator, which is one of the most prestigious honors in life sciences research, he said he thought of the many contributions former and current members of his lab have made over the years.
“Many of my scientific heroes are HHMI Investigators,” Flavell said. “The idea of being included in that group is humbling. But my main reaction is that I just feel extraordinarily proud of the current and former members of my lab. This is really their achievement.”