Memories are not just representations of “what.” They are also representations of when. Timing can be a crucial component of how we regard and make later use of our memories. If we remember experiences as events with characteristic sequences of steps, for instance, we can know what to expect in similar situations in the future. Over more than a decade of study the lab of Picower Professor Susumu Tonegawa has revealed important findings about how the brain accounts for timing and events in memory.
A pair of the lab’s studies examined the key role that a region called the medial entorhinal cortex (MEC) plays in infusing memory in the hippocampus with information about timing. In 2011 the lab dicovered that mice gained the ability to associate elements of an experience separated by time specifically because of a circuit connecting cells in layer 3 of the MEC with the hippocampus. Mice in whom the connection was disrupted, for instance, couldn’t properly learn from training in which a sound occurred 20 seconds before they received a little shock. Three years later the team refined this understanding, showing that “island cells” in layer 2 of MEC connect into the CA1 area of the hippocampus to control the activity of the connections from the MEC layer 3 cells. For instance, by engineering cells involved to be controlled by flashes of light (a technology called “optogenetics”) the scientists could manipulate whether mice could associate a sound with a shock and how much time could elapse between the two and still be associated in the mice’s memories.
The Tonegawa lab continued to examine the importance of timing, for instance by examining how the brain remembers sequences of events. In April 2020, they discovered a mechanism for the brain to store and use abstract representations of events, for instance to enable recognition of similar event sequences in other contexts. In CA1 of the hippocampus his team identified a distinct neuronal population of “event-specific rate remapping” (ESR) cells that, in communication with the MEC, encoded transferrable segments of experience (e.g. laps around a maze). Mice who ran laps came to expect a reward after a certain number of them, regardless of whether the laps were long or short, or the shape of the track. The ESR cells essentially just counted the laps. The finding suggested that the while the hippocampus encodes two independent codes about experience, the spatial and temporal details, it also more broadly represents abstract stages of experience (the concept of “a lap”).
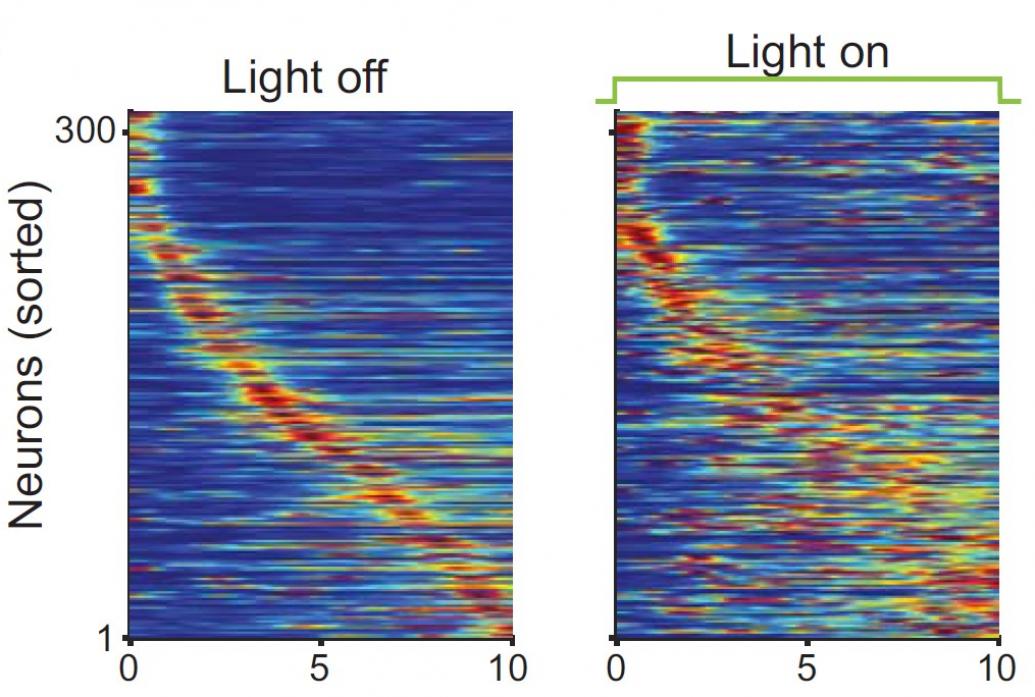
Above: Inhibition of input from the CA2 region of the hippocampus ("light on" condition on right) led to a much less organized neural response in the CA1 region to timing in a memory task.
A year later Tonegawa’s lab published another study that revealed how two hippocampus areas interact to encode sequences in time. The researchers identified a circuit between the CA2 and CA1 areas of the hippocampus that mice used to store information about the timing of when they should turn left or right in a maze. When the scientists blocked the circuit, the mice couldn’t remember which way they were supposed to turn next. Notably, disrupting the circuit didn’t impair their recollection of their location. That showed that the circuit was specific to remembering timing of what to do next.

