Studies of consciousness often run into a common conundrum of science—it’s hard to measure a system without the measurement affecting the system. Researchers assessing consciousness, for instance as volunteers receive anesthesia, typically use spoken commands to see if subjects can still respond, but that sound might keep them awake longer or wake them up sooner than normal. A new study not only validates a way to assess consciousness without external stimulation, it also finds that it may be more precise.
“We want to measure when people make the transition from conscious to unconscious, and vice versa, but as soon as you ask someone to do something, which is the classic way of assessing this, you’ve now influenced them and disrupted the process,” said Christian Guay, lead author of the study in the British Journal of Anaesthesia. Guay is a research collaborator at the Neuroscience Statistics Research Laboratory in The Picower Institute for Learning and Memory at MIT, and an anesthesiologist and critical care fellow at Massachusetts General Hospital (MGH). “We think that conscious state transitions are interesting because they are very dynamic in the brain, but the neural mechanisms mediating these transitions aren’t fully understood, in part because of how we are assessing the transitions.”
Moreover, Guay is part of a collaboration with co-authors and former colleagues at Washington University in St. Louis to test whether a method of closed loop acoustic stimulation can augment the effects of dexmedetomidine-mediated sedation. For that reason, too, they needed a method of assessing consciousness that didn’t require sounds that could confound the results.
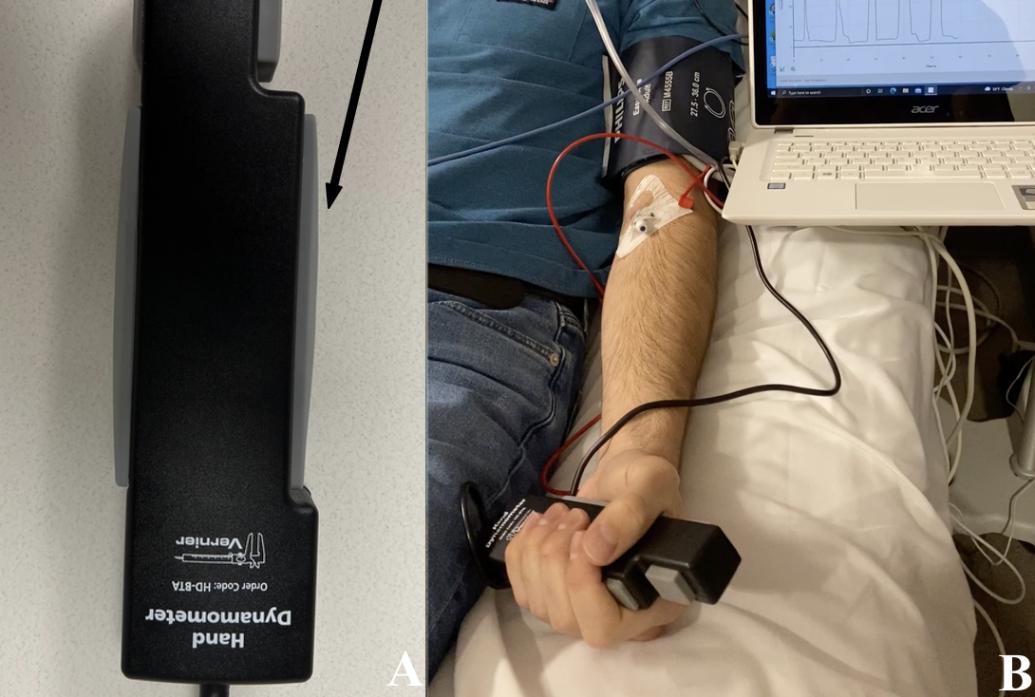
So the team found a different, little-used approach first described in 2014 by sleep researchers. Before the infusion began, they instructed their 14 volunteers to squeeze a force sensor with their hand whenever they breathed in and release it when they breathed out. Then the drug started flowing. When subjects stopped performing the “breathe-squeeze task,” they were judged to have lost responsiveness and when they resumed after dosing tapered off, they were judged to have regained responsiveness. Importantly, after the initial instruction there was no ongoing external stimulation from the researchers. The task was internally prompted.
Above: A figure from the study shows the experimental setup. Volunteers would squeeze and release a force sensor as they breathed. Image courtesy Christian Guay.
All along, the researchers recorded the subjects’ brain rhythms using 64 electrodes around the scalp. They observed telltale patterns of dexmedetomidine effects—for instance a decline in ~10Hz “alpha” rhythm power in the occipital region followed by an increase in power of much slower “delta” waves as people lost responsiveness and then a reversal of that when they woke up. Because of their approach they didn’t see artifacts of auditory stimulation that disrupted those patterns in a previous study that used sound to measure consciousness in people receiving the same anesthetic. Moreover, estimates of drug concentration in the brain during the two studies suggest that the breathe-squeeze method detected loss of responsiveness at lower concentrations of the drug than the sound-stimulation method, suggesting it is more sensitive.
“This approach for assessing loss and recovery of consciousness removes the significant confound of the conventional external stimulus that is typically used,” said study co-senior author Emery N. Brown, Edward Hood Taplin Professor of Medical Engineering and Computational Neuroscience in The Picower Institute at MIT as well as an anesthesiologist at MGH and Warren M. Zapol Professor of Anaesthesia at Harvard Medical School. “We are eager to apply the technique in our studies of other anesthetics.”
At MIT and MGH, Brown is leading a new initiative, the Brain Arousal State Control Innovation Center (BASCIC), to better unify anesthesiology and research into the neuroscience of the brain’s arousal systems so that they can each inform and improve each other, and spawn new clinical innovations. Guay, who is a member of the effort, notes that as researchers achieve a better understanding of the transition from consciousness to unconsciousness, they could help treat insomnia better, and if they understand the process of waking better they might be able to improve the chances of coma reversal. Improving methods of assessing consciousness transitions are key to those efforts.
In addition to Guay and Brown, who is a faculty member in MIT’s Department of Brain and Cognitive Sciences and Institute for Medical Engineering and Science, the study’s other authors are Darren Hight, Guarang Gupta, MohammadMehdi Kafashan, Anhthi Luong, Michael Avidan and Ben Julian Palanca.
Funding for the study came from the McDonnell Center for Systems Neuroscience at Washington University. Brown’s MIT lab is supported in part by The JPB Foundation.