“The brain requires a diversity of mechanisms at its disposal in order to adapt to stimuli. We know that neurons have different properties, and we demonstrated several years ago that neurons have different patterns of myelination. Now, we have found an additional layer of complexity: neurons actively use their myelin in dynamic and different ways,” said co-senior author Paola Arlotta, the Golub Family Professor of Stem Cell and Regenerative Biology at Harvard University.
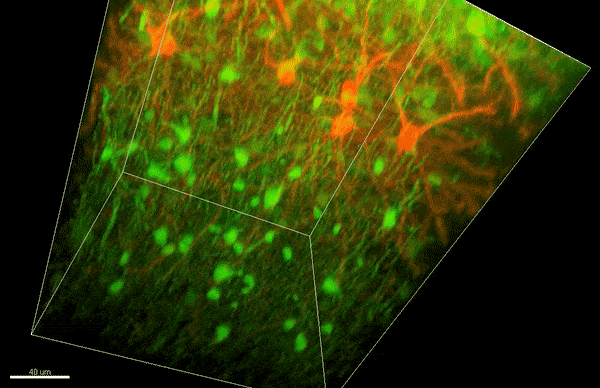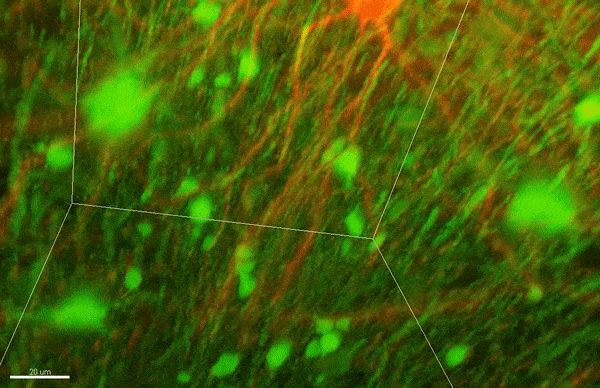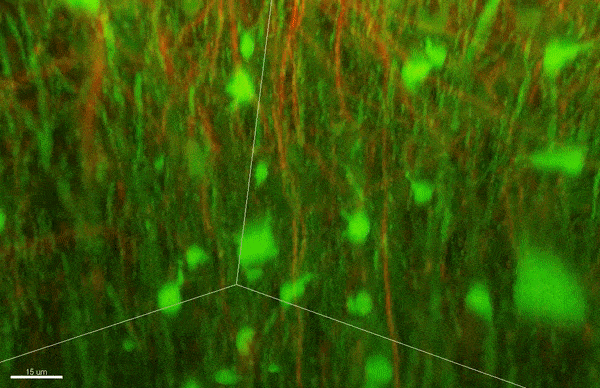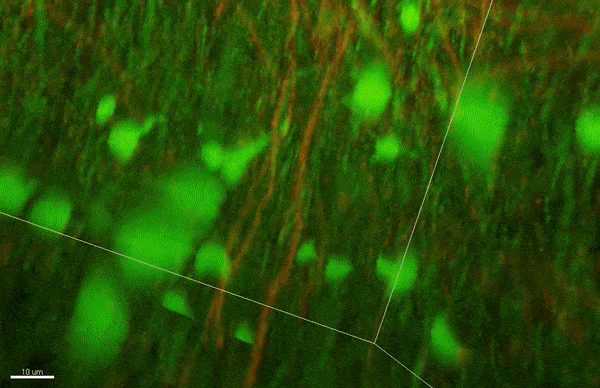
In biology textbooks, the prototypical image of a neuron depicts the axon with a series of equally sized, evenly spaced pieces of myelin. However, the Arlotta lab showed in 2014 that the picture is more complicated: different types of neurons show different patterns of myelination, with varying lengths of myelin or no myelination on some segments. In the current study, the researchers delved deeper into the phenomenon of how myelination patterns might change over time.
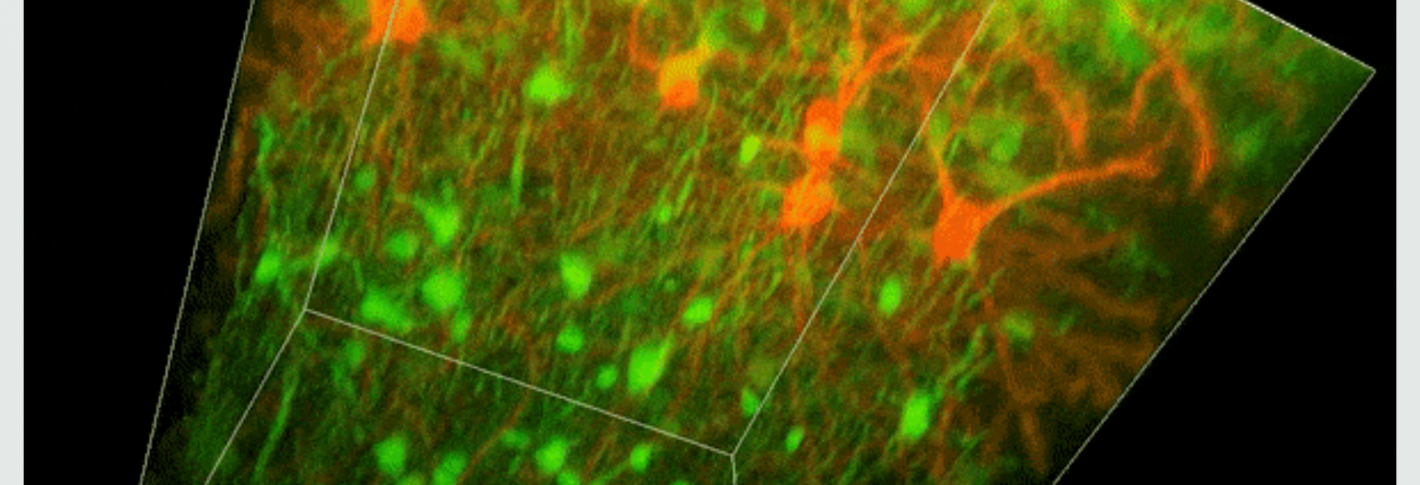
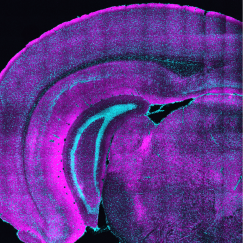
To investigate myelin plasticity, the researchers used mouse models where specific neuron types were fluorescently labeled. They changed the animals’ sensory input by closing one eye, then tracked how the brain responded using a custom-built in vivo imaging system in the lab of collaborator and co-senior author Elly Nedivi.
“Our multicolor method enables the simultaneous visualization of both the myelin and the axons it was wrapping. This allowed us to closely track how myelin was changing over time as mice reconfigured the visual cortex as sight became deprived in one eye,” said Nedivi, who is the William R. (1964) & Linda R. Young Professor of Neuroscience at MIT, and a member of The Picower Institute for Learning and Memory and the Departments of Biology and Brain and Cognitive Sciences.
The researchers found that even though they tracked neurons that were next to each other and part of the same network, different cell types had different responses — specifically, inhibitory neurons remodeled their myelin more than excitatory neurons.
“In the inhibitory neurons, we saw a two-times increase in the number of myelin changes. Those changes can be any way you can imagine: they can be myelin segments shortening or elongating, the addition of new myelin, and also the elimination of an entire piece of myelin,” said Sung Min Yang, lead author and postdoctoral fellow in the Arlotta lab.
The unique capacity to change their myelin opens up possibilities for the neurons, Yang said: “It turns out that neurons do not move myelin around in a consistent way, as in a game of checkers where every game piece has the same move. Instead, the brain is playing chess, where different neurons — or pieces — can move in different ways. This gives the brain more choice in how to use myelin, which is a limited resource.”
The researchers also found that neurons did not necessarily have to produce new myelin, but could reuse and reshape what they already had in order to respond.
“We have discovered a fundamental property of the brain that opens the window to conceptualizing how the organ maximizes its power and optimizes its function. The dynamic distribution of myelin is yet another level of mechanism that the brain uses to diversify its response to a given stimulus — the endless combinations can enable a more complex, even surprising outcome,” Arlotta said.
Based on the findings, the researchers can now investigate how myelin plasticity plays a role in other contexts, including disease.
“We hope to be able to investigate myelin pathology in human brain organoid models, which can be generated from patients or engineered to contain specific mutations associated with myelin abnormalities, in order to better understand the disease mechanisms,” Arlotta said.
This research was supported by the Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard, the National Institute of Mental Health, the National Institutes of Health, and the JPB Foundation.
--Text adapted from Harvard University