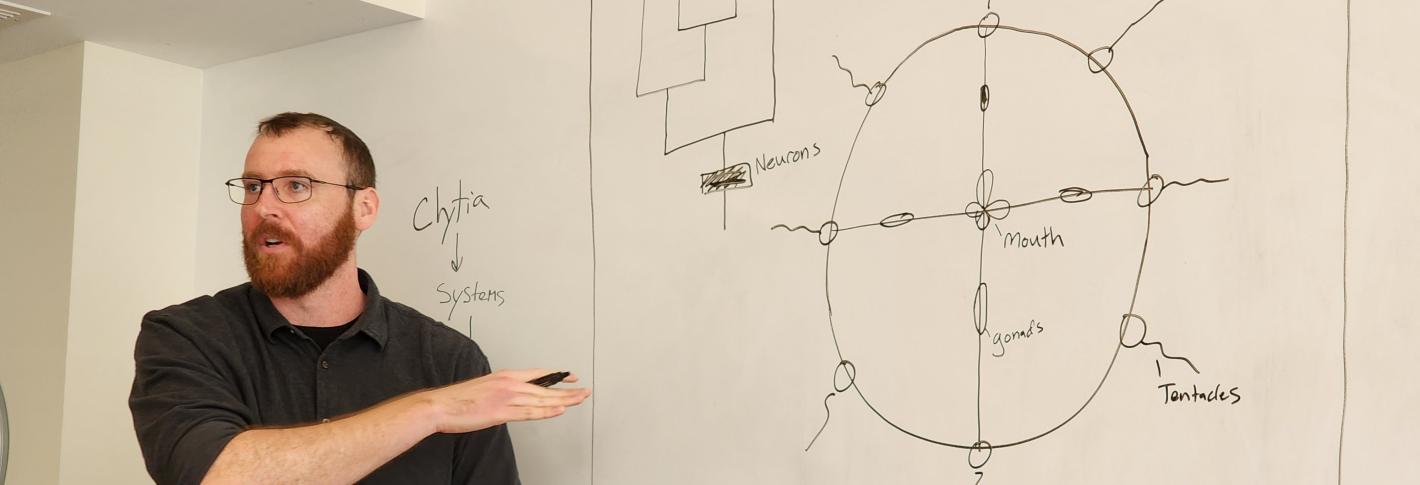
Scientists who dream of a future in which regenerative medicine has advanced enough to enable repairs in human nervous systems currently have more questions than answers. As a recently named Searle Scholar, MIT Assistant Professor Brady Weissbourd will seek to learn some of the needed fundamentals by studying a master of neural regeneration: the jellyfish, Clytia hemisphaerica.
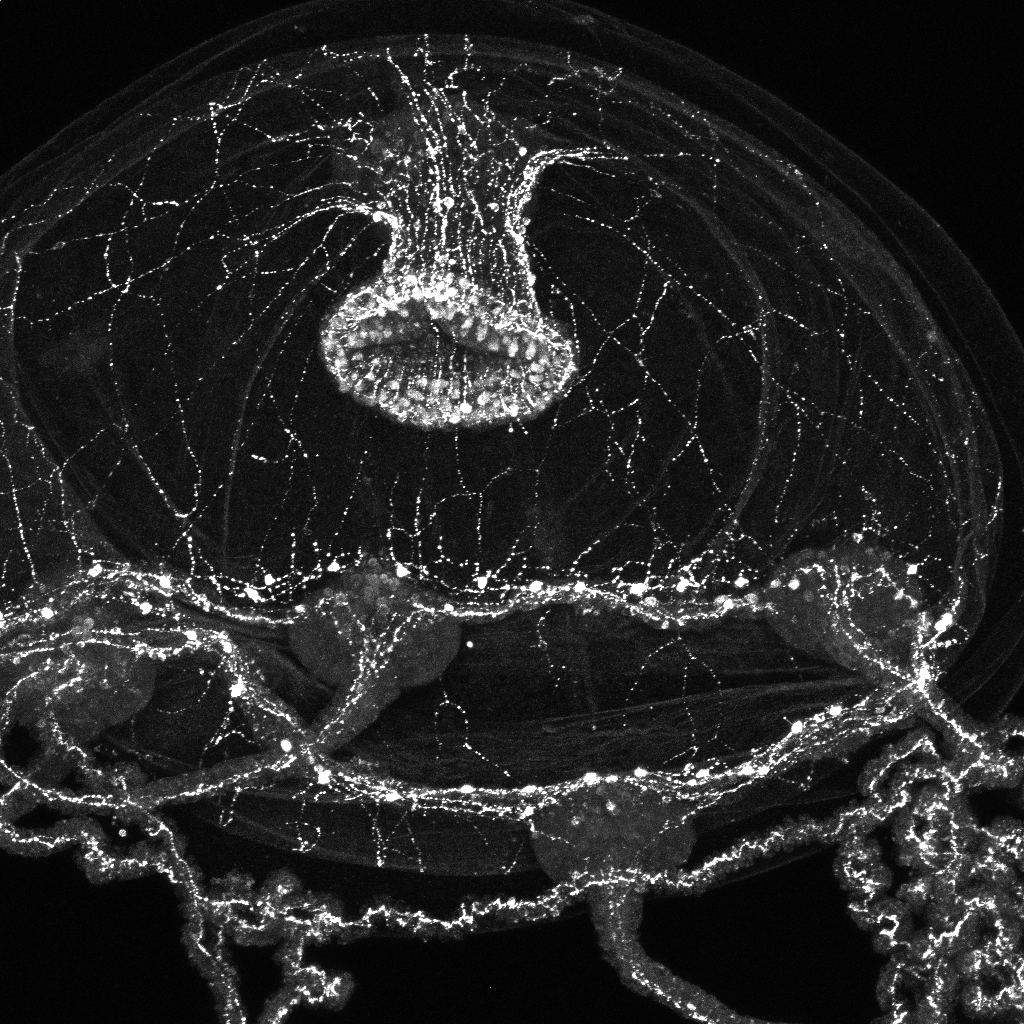
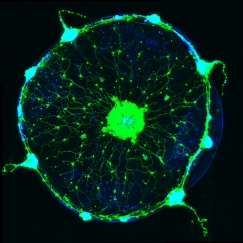
Weissbourd, a faculty member in the Department of Biology and The Picower Institute for Learning and Memory, has helped to pioneer use of the seafaring species in neuroscience research for many reasons. It is transparent for easy imaging, reproduces rapidly, and shares many basic nervous system properties with mammals despite diverging evolutionarily 600 million years ago (just after the development of the earliest nervous systems). Meanwhile, with about 10,000 neurons, the jellyfish fills a gap in the field in terms of that degree of complexity.
But what Weissbourd didn’t appreciate until he began experimenting with the jellyfish was that they are also incredibly good at refreshing and rebuilding their nervous systems with new cells. After becoming the first researcher to develop the ability to genetically manipulate the organism, he started teasing out how its highly distributed nervous system (there is no central brain), was organized to enable its many behaviors. When he ablated a subnetwork of cells to test whether it was indeed responsible for a particular feeding behavior, he found that within a week it had completely regrown. Moreover, he has observed that the jellyfish constantly produce and integrate new cells, even in the absence of major injury.
Looking for the logic
The finding raised a proverbial boatload of intriguing questions that his support of $100,000 a year for the next three years from the Searle Scholars Program will help him pursue.
“Where are these newborn neurons coming from in both the normal and regenerative contexts?” Weissbourd asked. “What rules guide them to the correct locations to rebuild these networks, both to integrate these newborn neurons into the network without messing it up and also to recreate it during regeneration? Are the rules the same or different between these contexts?”
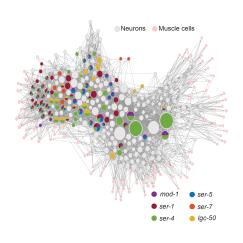
Additionally, by using a combination of techniques such as imaging neural activity during behavior, sequencing gene expression cell by cell, and computational modeling, Weissbourd’s lab has discerned that within their web-like mesh of neurons, jellyfish harbor more than a dozen different functional subnetworks that enable its variety of different behaviors. Can all the subnetworks regenerate? If not, why do some forgo the remarkable ability? Among those that do regenerate, do they all do so the same way? If they employ different means, then learning what those are could provide multiple answers to the question of how new neurons can successfully integrate into existing neural networks.
Building on support provided by a Klingenstein-Simons Fellowship Weissbourd earned last year, he’ll be able to pursue experiments designed to understand the “logic” of how jellyfish manage neural regeneration.
“The ability to understand how nervous systems regenerate has significant implications for regenerative medicine,” Weissbourd said.
A complete 3D ‘wiring diagram’
As part of the new award, Weissbourd also plans to create a major new resource for jellyfish neurobiology to advance not only this project, but also the research of any other scientist who wants to study the organism. Working with collaborator Jeff Lichtman, a professor of molecular and cellular biology at Harvard University, Weissbourd will create a complete 3D reconstruction of a jellyfish’s nervous system at the subcellular resolution enabled by electron microscopy. The resource, which Weissbourd plans to provide openly online, will amount to a full “wiring diagram” of a jellyfish where every circuit connection can be mapped.
Being able to see how every neural circuit is constructed in a whole animal will enable Weissbourd to answer questions about how the circuits are built and therefore how new neurons integrate. Having a complete and detailed view of every circuit will improve the computational models his lab is building to predict how anatomy helps give rise to function and behavior. And given that new neurons are being born, migrating and integrating all the time, Weissbourd said, the imaging will also likely yield a snapshot of neural regeneration in action in its many stages.
Weissbourd said he was grateful for the honor of being named a Searle Scholar, which not only provides support for his lab’s work, but also welcomes him into a new community of young scientists.
“I’m honored and super excited,” Weissbourd said. “I’m excited to interact with the other scholars as well.”