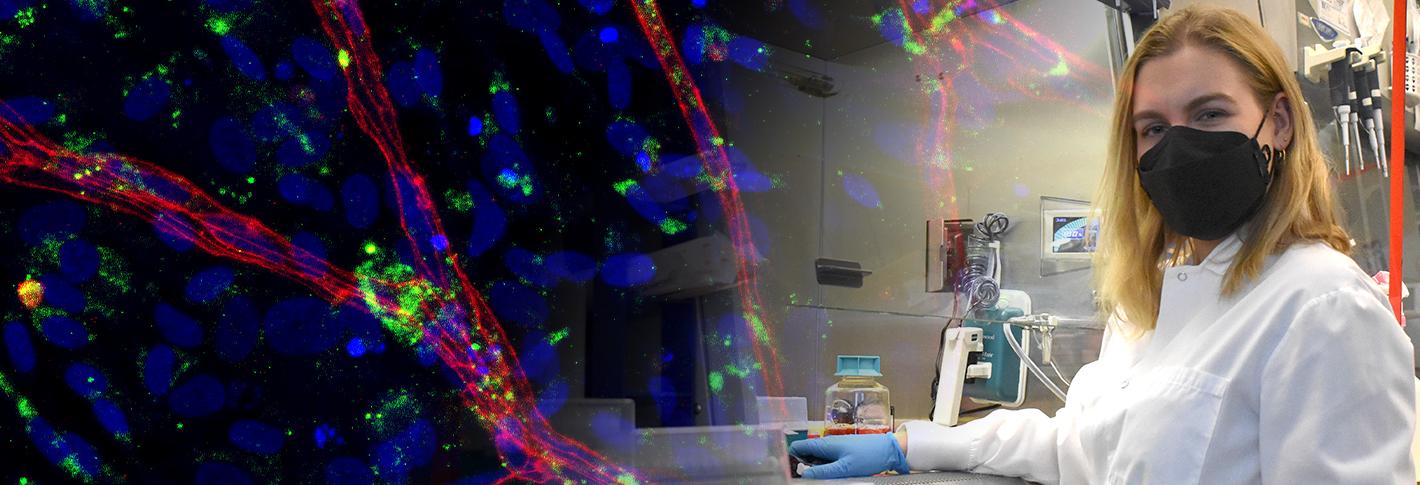
The advent of human induced pluripotent stem cells (iPSCs) has heralded a new generation of clinical and basic research into human disorders. Patient-derived skin fibroblast cells are reprogrammed into iPS cells, which allow researchers to directly examine a wide variety of diseases directly in human cells in addition to studying gene variants in patient populations.
Launched in November of 2010, the iPS Core Facility was created to support researchers from the Picower and McGovern Institutes and the Department of Brain and Cognitive Sciences in creating human and animal cell models of diseases. The various laboratories have expertise and experience with different experimental protocols which, when combined in a collaborative manner to the study of human cells, result in accelerated progress in this novel, dynamic and competitive field.
Since its inception, the facility has been used by more than 30 researchers at MIT and has rapidly become essential to studies of autism, psychiatric disease, Alzheimer’s, and many neurodegeneration diseases. More than 90 researchers have received the iPS cell culture training.
Effective FY14 the iPS facility opened its doors for the first time to other MIT users and to users external to MIT as a fee for service facility. Collaborations with researchers outside MIT have been increasing and include noteworthy interactions with the Broad Institute and other biotech industry representatives.
The facility offers an orientation and training program and is accessible to users at all hours with a keycard security system. We can provide generation of human iPS cells and CRISPR modification of iPS cells. Shared equipment is available with a sign-up reservation system.
Equipment and capabilities
The iPS Core Facility is equipped for the specialized production, maintenance, expansion, preservation, and distribution of human fibroblasts, iPS cell lines, iPS- and ES-derived neuronal progenitor cells, iPS-and ES- derived neurons, induced neuronal (iN) cells, and neural organoids.
The iPS Core Facility offers:
- Five rooms in Buildings 46 and 47 encompassing more than 2,500 square feet of space in four tissue culture areas; one room is dedicated for viral work with iPS ES cells as BL2plus practice for higher safety protocol, and three tissue culture areas are for maintenance, expansion, and general handling of non-viral work related iPS and ES cell culture and neuronal differentiation with BL2 safety practice.
- 20 bio-safety cabinets, 33 CO2 incubators, and one three -gas incubator that allows controlling of O2 concentration, and bench areas.
- A MELODY FACS machine is also available for live cell sorting in another room of our facility.
- Five bio-safety cabinets equipped with microscopes for observation and handling of cells in a clean and protected environment.
As of 2023, the iPS Core Facility has produced more than 90 patient-specific iPS cells from people with schizophrenia, bipolar disorder, depression, Rett syndrome, Alzheimer's disease, Down syndrome, and healthy person’s skin fibroblasts as controls. Some of these cell lines are “isogenic” meaning they differ only in exact, specified genetic changes that allow for direct comparisons (for example, APOE3 vs. APOE4 Alzheimer’s risk gene variants). The number of cell lines and iPS cells continue to grow.
The iPS core also provides for fee service for outside entities. To learn more, contact the iPS Core Facility Supervisor, Tak Ko.