For the brain to produce its many functions, neurons must communicate. They do this by releasing chemicals called neurotransmitters across connections called synapses. The diversity of the brain’s capabilities is, in many ways, a result of the wide variety of synapses and the ability of neurons to adjust how each of them operates. More than two decades of research by the Picower Institute lab of Menicon Professor Troy Littleton have yielded numerous discoveries about how synapses work and the nature of their variance and flexibility.
The trigger for one (“presynaptic”) neuron to communicate with a downstream (“postsynaptic”) partner is a peak of voltage called an action potential that causes calcium ions to surge into the presynaptic side. One of Littleton’s first discoveries at MIT, building on his graduate and postdoctoral work, was that a protein called synaptotagmin 1 (Syt1) senses that calcium and plays a key role in synchronizing fusion of vesicles containing the neurotransmitter glutamate to the outermost presynaptic membrane so their contents can be released across the synapse.
Syt1, which is attached to the vesicles, does not work alone. Littleton and his lab have helped to show and describe how presynaptic neurons assemble a protein complex made up of SNAREs, which binds with Syt1, to facilitate vesicle fusion and regulate release timing. Other work by the lab has shown how another key protein called Complexin coordinates with Syt1 and acts like a clamp to ensure that vesicles don’t fuse prematurely, for instance without Syt1 sensing an influx of calcium ions.
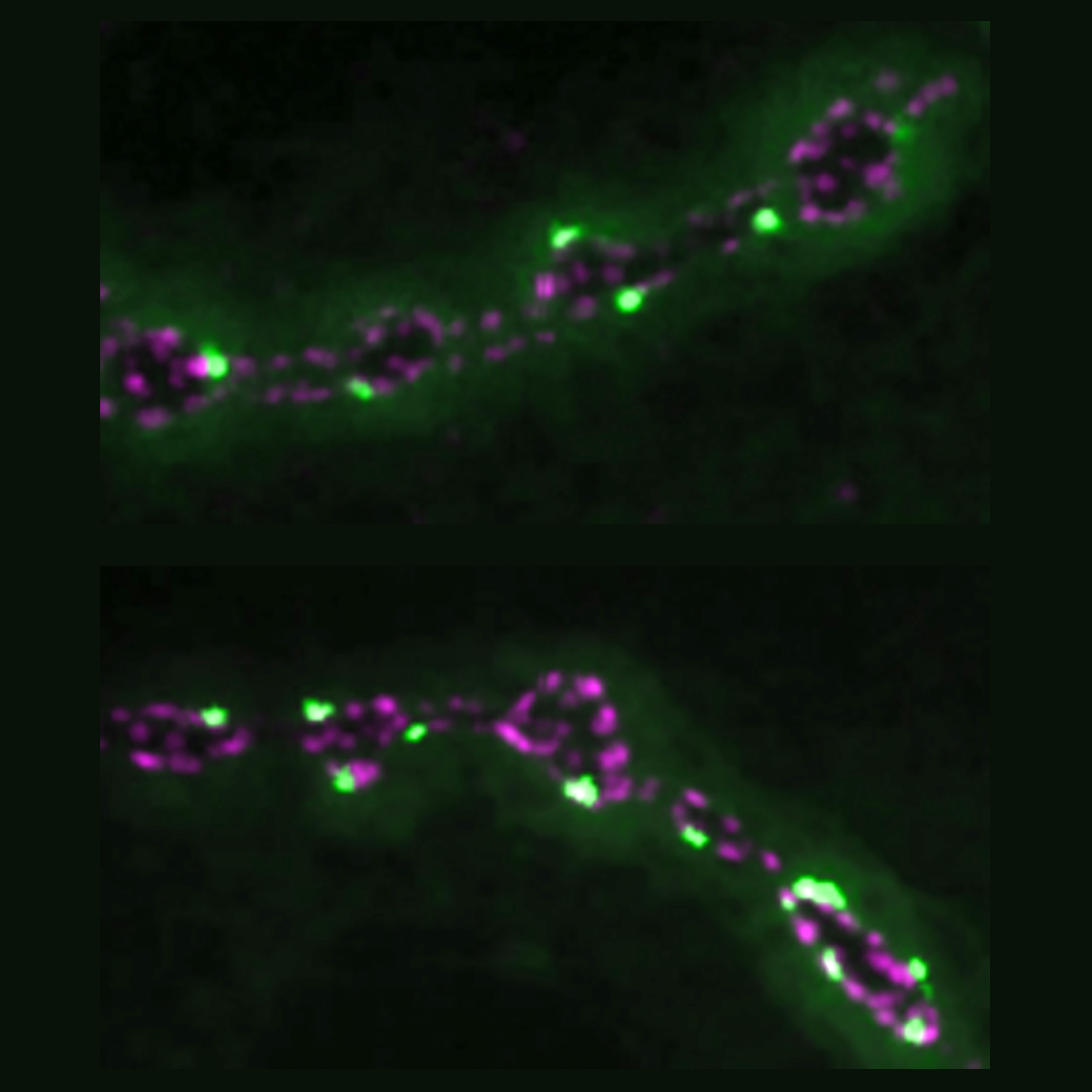
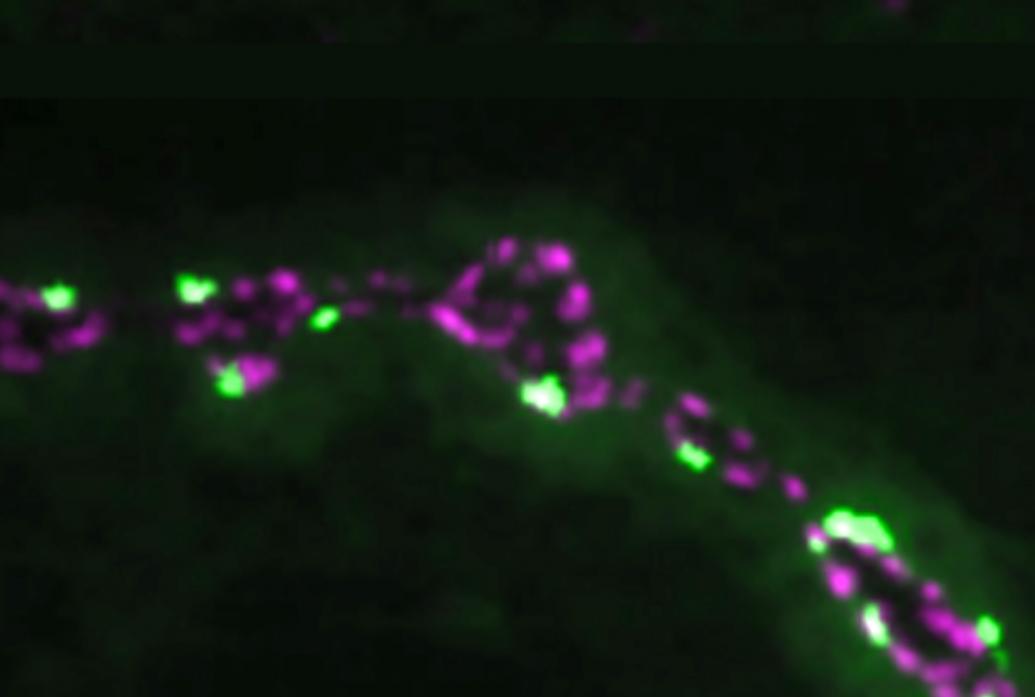
Other Littleton Lab studies have found mechanisms providing additional degrees of control over the timing and amount of neurotransmitter released. In 2013, the team showed that while many of release sites called active zones are used both for release evoked by action potentials and others that occur spontaneously, the two kinds of neurotransmitter release are regulated independently. In 2018, Littleton’s lab used innovative imaging methods to find that some active zones develop to become up to 50 times stronger than others within the same neuron’s array of synapses. The difference, they found, owed to the abundance of ion channels that allowed for the flow of calcium and the amount of a scaffold proteins that help cluster those channels and the associated synaptic vesicles containing neurotransmitter. The organization of proteins on the postsynaptic side mattered, too. And in 2020 Littleton Lab members discovered that Synaptotagmin 7 (Syt7) regulates the amount of neurotransmitter vesicles available for release. The more Syt7, the tighter the supply. Changing the levels of Syt7 therefore is essentially like turning a dial to control how strongly communication occurs across a synapse.

