A defining feature of the brain is that it is not “hardwired.” Instead, the connections between neurons called synapses can change in response to neural activity, making the brain flexible and adaptable (a property called “plasticity”). If it weren’t, we couldn’t learn or remember anything new. Many studies in the lab of Menicon Professor Troy Littleton have revealed ways in which synapses are built to change and how both the “pre-” and “post-” synaptic sides of a synapse interact to set plasticity in motion.
In 2005, for example, Littleton and lab members discovered the means by which activity in a postsynaptic neuron can signal to the presynaptic side to grow stronger. Such “retrograde signaling,” they found, was mediated by a protein called Synaptotagmin 4 (Syt4). When the postsynaptic neuron is very active and calcium ions flow in, Syt4 senses that and signals the presynaptic neuron to emit a bunch of small neurotransmitter releases (called “minis”) that strengthen the connection.
In 2011 the lab looked at how incoming growth signals are transported within the presynaptic side, identifying the proteins Nervous Wreck and SNX16 as key means of trafficking these messages to their destinations to promote growth and to subsequently turn the growth signal off. On the postsynaptic side they showed in 2016 that the protein Syntaxin4 (Syx4) is a critical regulator of how Syt4 works to stimulate retrograde signaling and how other proteins contribute to synapse growth. And in 2015 the team explained how Syt4-mediated postsynaptic signals cause the presynaptic side to emit “minis.” Normally presynaptic neurotransmitter release is regulated by a clamp-like protein called Complexin. But the Syt4-related signals stimulate a protein called PKA to chemically alter Complexin, allowing mini release to occur.
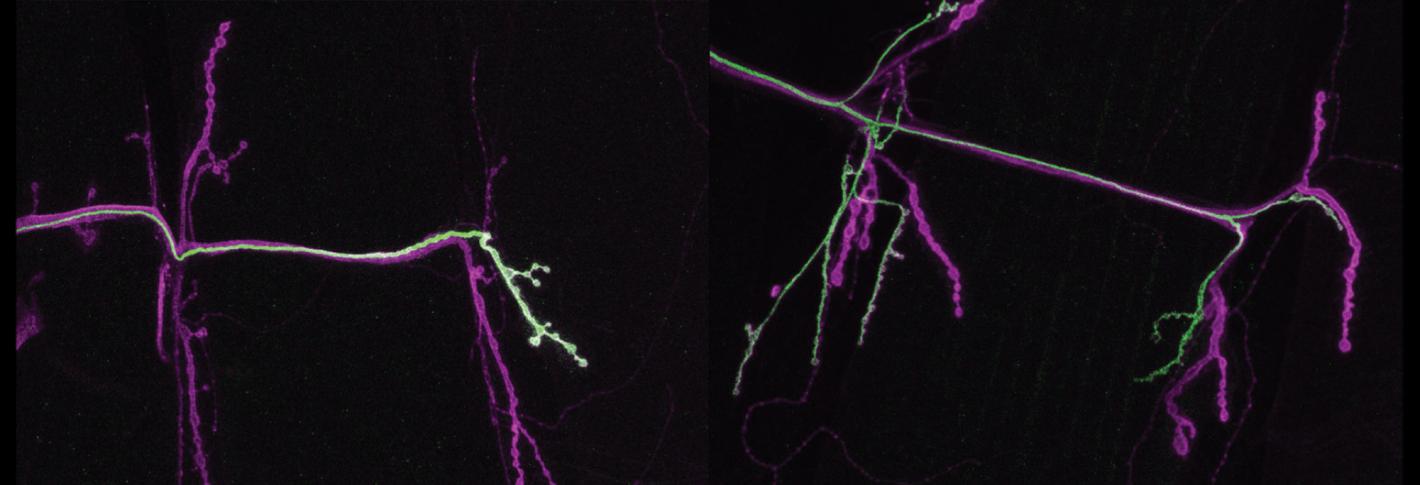
Above: The left panel shows the "tonic" neuron (stained green) innervating just one muscle on the right of the panel. In the next panel, one can see the phasic neuron (also stained green) connecting to more than one muscle.
In parallel studies, Littleton’s Lab has also identified how neurons are differently built to change, or not to change, their activity. In 2020, they showed that two seemingly similar neurons that innervate the same muscle differ in their ability to engage in plasticity. Phasic neurons, which emit big bursts of the neurotransmitter glutamate, don’t robustly change their behavior, while tonic neurons, which emit lower but more constant levels of glutamate, can step up that pace if phasic neurons go offline. In 2021 they discovered a structural reason for the functional difference. Tonic neurons express a protein called tomosyn that helps them regulate neurotransmitter release, so they always have some in reserve if they need to suddenly do more. Tomosyn works by intercepting glutamate vesicles, binding to them before they can bind to the SNARE protein complex that would bring them to the synapse membrane to be released. Phasic neurons have less Tomosyn and therefore fail to conserve their glutamate supply when release is stimulated.

