The Drosophila melanogaster fruit fly is clearly different than a person but the basic operation of its nervous system, and the genes and proteins involved, are closely analogous—they have been “conserved” throughout evolution. Because of these fundamental parallels, Drosophila can powerfully and accessibly model many human diseases. The Picower Institute lab of Menicon Professor Troy Littleton has made several discoveries about human diseases by studying the fruit fly nervous system.
Huntington’s disease is a terminal neurodegenerative condition resulting from a mutation in the Huntingtin gene. Littleton’s lab has been studying the effects of loss of normal Huntingtin function in Drosophila models since 2009, yielding a series of studies providing insights into the disease. In 2012 the lab demonstrated and imaged how the mutant protein harmfully misaggregates. They’ve found loss of normal function causes defects in the transport of mitochondria and neurotransmitter vesicles within neural axons. They also discovered that mutant Huntingtin can disrupt intracellular signaling governing the growth of neural connections, or synapses, leading to excessive growth.
Autism spectrum disorders may at least in part be the result of defects in the way that neurons connect at junctions called synapses. In a 2016 study of the autism-linked gene Shank3, which encodes a protein that helps to build developing synapses on the post-synaptic side, Littleton’s team reported multiple problems in synapses when Shank was knocked out in fruit flies. Receptors for a key form of molecular signaling from the presynaptic side called Wnt failed to be internalized by the postsynaptic cell, meaning they could not influence the transcription of genes that promote maturation of the synapse as they normally would. To set the stage for potential drug development, Littleton’s lab was able to demonstrate ways to bypass Wnt signaling that rescued synaptic development.
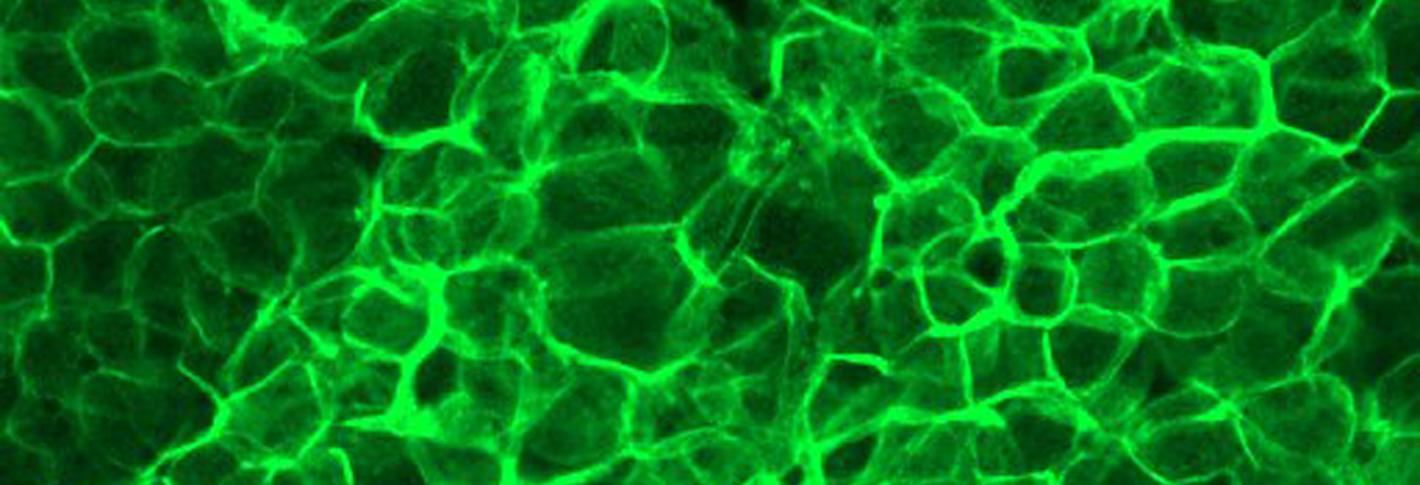
Above: A green glow indicates elevated activity of the protein calcineurin in glial cells.
Epilepsy is characterized by seizures that result from hyperexcitability of neurons. In 2005 the Littleton Lab discovered a seizure-causing mutation on the X chromosome that they called Zydeco. In 2013 they showed that the mutation caused non-neural “glial” cells to retain too much calcium. By 2019 they found out how that led to seizures. The excess of calcium overactivated a protein called calcineurin. That caused the glial cells to withdraw a means of removing potassium ions from the area around neurons. That, in turn, meant neurons couldn’t get rid of excess potassium, leaving them electrically overexcited and prone to seizures. The research therefore provided a way to potentially target seizures without having to alter neurons directly.

