A Systems Approach
Over the past five years, impressive advances in technologies have enabled us to more fully explore the brain and its complex circuitry of neurons. We now have better ways to see deep inside the brain and trace neural networks in unprecedented detail along with methods that allow us to stimulate specific neurons using laser light. These new tools have enabled researchers to more precisely study the neural circuits and brain activity patterns that encode our memories, translate our movements, and predict our decisions.
Restoring Memories
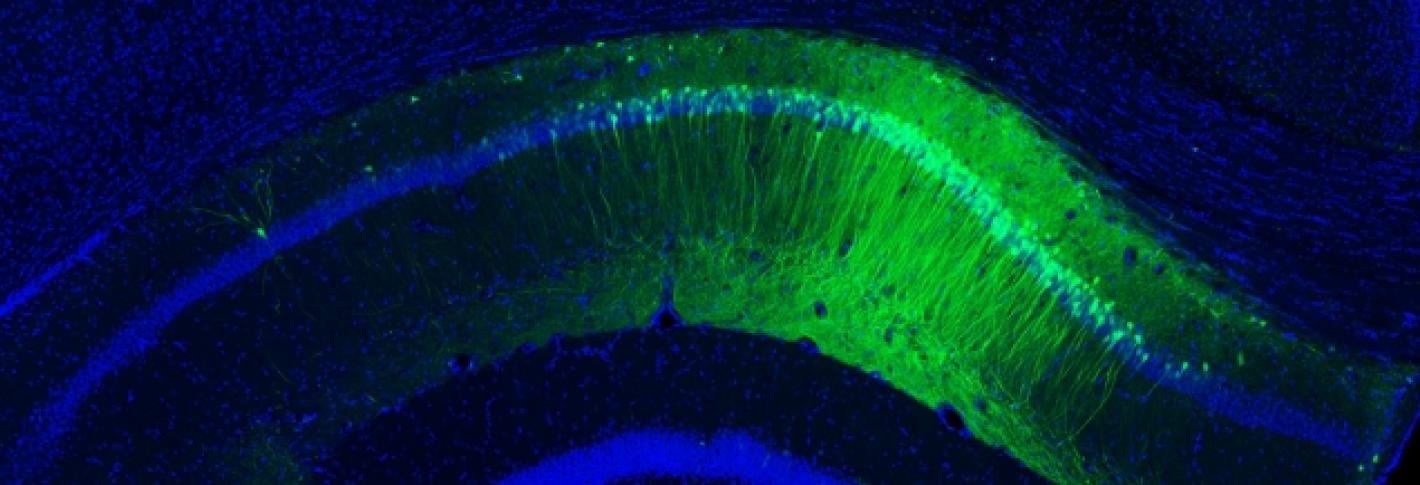
MIT’s Picower Professor Susumu Tonegawa, who studies memory, discovered how to identify the specific brain cells that encode a particular memory. He also developed a way to stimulate specific brain cells at specific times by inducing a light-sensitive protein within them and then pulsing them with laser light. In experiments with mice in the early stages of Alzheimer’s disease, Tonegawa’s team was able to use these techniques on memory cells to trigger them to recover short-term memories, such as a fear of a particular place, which the mice had forgotten. This research suggests strongly that memory loss in Alzheimer’s disease is actually caused by a loss of access to memories and not the loss of the memory itself. Even when affected by dementia, Tonegawa found, the brain still has the ability to learn and to store a memory.
Tonegawa and his team also found that strengthening the synaptic connections of those memory cells enabled longer-term restoration of the lost memories. Knowing that memory reactivation is possible, MIT researchers such as Boyden and Tsai are exploring noninvasive ways, such as using electricity or magnets transcranially to target deep and specific locations in the brain, to stimulate memory cells in human patients.
Improving Cognition
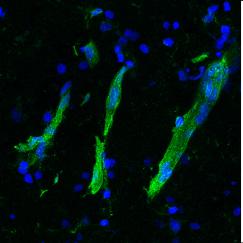
Where in the brain does Alzheimer’s disease begin? Using the new-tissue clearing methods to examine neural circuits in unprecedented detail, Tsai in collaboration with assistant professor Kwanghun Chung have found that the first signs of degradation occur in deep brain regions that are involved in brain-wide memory processing. These actions may then trigger a cascade that affects the circuits that store short-term memories of everyday events. Tsai’s team is now tracing how these brain networks decline. Because memory circuit malfunction is likely to be a primary cause of cognitive decline, brain stimulation therapies—including brain wave stimulation—may be able to restore healthy network patterns and improve memory and cognitive capacity, while also potentially modifying the course of the disease.
Novel drug therapies may be another approach to restoring memories. In mouse experiments, Tsai’s team used molecules that inhibit enzymes known to regulate gene expression involved in forming memories. These drugs can turn on genes that had otherwise been blocked, and are able to bring back “lost” memories in mice with Alzheimer’s disease. The researchers are now exploring whether this approach will be effective in treating Alzheimer’s disease or other degenerative diseases in humans.
Biomarkers of Unhealthy Aging
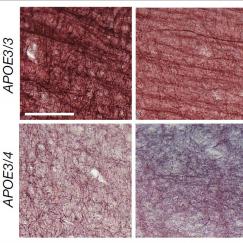
Neuroscientists face a critical knowledge gap—they don’t know why some people age well and others do not, or how their brains differ in their aging process. One promising strategy to bridge this gap is to map what is known as the human epigenome—in effect, to map which genes are turned on in which cells in all parts of the body. This effort is part of a national project that MIT and professor Manolis Kellis ’99, MNG ’99, PhD ’03, are leading, and which will collect massive amounts of data from many different individuals. By applying MIT’s strength in big data strategies and artificial intelligence “deep-learning” techniques to this data, Kellis, in collaboration with Novartis Professor of Biology Leonard Guarente ’74 and Tsai hope to identify key biomarkers for healthy and unhealthy aging. Such biomarkers would enable both early diagnosis and ways to track the progression of disease or improvements from therapies. Already, Kellis and Tsai have found a biomarker in the regulatory area of the brain’s immune genes that is a major risk factor for Alzheimer’s disease.
Measuring Brainwaves for Early Diagnosis
Anesthesia affects the brain differently as people age. MIT’s Emery Brown, the Edward Hood Taplin Professor of Medical Engineering and of Computational Neuroscience—who is both a computational neuroscientist and an anesthesiologist—uses EEG, a technology that uses electrodes to record brain waves, to show that patterns and strength of brain waves change with age. He has characterized differences in brain waves in patients under anesthesia between the ages of 18 and 90 years, and is using these data to create a brain-age calculator. The technique has the potential to analyze differences in patients with and without Alzheimer’s disease, or to gauge how well an individual’s brain is aging, and thereby help identify brains whose wave patterns suggest they may be at a higher risk of developing Alzheimer’s disease, even well before the cognitive decline and other behavioral symptoms appear.
Bringing New Tools and Therapies to Market
Research is powerful. But discoveries only benefit people once the products from discovery are approved by regulatory agencies and become commercially available. Because MIT anchors what is arguably the biotech capital of the world—an enormous concentration of biotech companies and research centers—it can rapidly translate new discoveries into practical applications ready for the marketplace. MIT’s location also means that the Aging Brain Initiative can collaborate with—and leverage skills and talent from—a wide array of partners. As a result, the members of the Aging Brain Initiative are confident that their research will go a long way toward halting or significantly slowing the progression of Alzheimer’s disease and other dementias and improve cognitive outcomes in those afflicted with these conditions.
To Make a TrANSFORMATIONAL Gift
OR FOR MORE INFORMATION , Contact:
Asha Bhakar, PhD
Director of Development
abhakar@mit.edu
(617) 258-0759